化学学报 ›› 2023, Vol. 81 ›› Issue (10): 1341-1349.DOI: 10.6023/A23060292 上一篇 下一篇
所属专题: 庆祝《化学学报》创刊90周年合辑
研究论文
投稿日期:2023-06-16
发布日期:2023-08-15
作者简介:基金资助:
Yang Wang, Junjun Xiang, Congwu Ge, Xike Gao( )
)
Received:2023-06-16
Published:2023-08-15
Contact:
*E-mail: About author:Supported by:文章分享
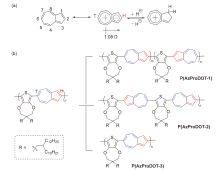
本工作通过理性分子设计和直接芳基化聚合策略, 合成了三个基于2,6-薁和3,4-丙撑二氧噻吩的共轭聚合物P(AzProDOT-1)、P(AzProDOT-2)和P(AzProDOT-3), 其主链中薁单元偶极取向排列方式分别为无规、部分规整和高度有序. 通过密度泛函理论(density functional theory, DFT)计算、紫外-可见吸收光谱和循环伏安法等对三个聚合物的物理化学性质进行表征, 三个聚合物在溶液和薄膜状态均表现出质子响应性质. 通过溶液旋涂法构筑了三个聚合物空间电荷限制电流(space-charge-limited current, SCLC)器件, 薁单元偶极取向排列高度有序的聚合物P(AzProDOT-3)表现出最优的器件性能, 空穴和电子迁移率分别为1.41×10−4 cm2•V−1•s−1和1.66×10−5 cm2•V−1•s−1. 本工作精准调控了2,6-薁共轭聚合物的主链结构, 为薁基共轭聚合物的合成化学和明晰其结构-性质/性能关系提供了研究思路.
汪洋, 向焌钧, 葛从伍, 高希珂. 2,6-薁和3,4-丙撑二氧噻吩共聚物的主链结构调控及性质研究★[J]. 化学学报, 2023, 81(10): 1341-1349.
Yang Wang, Junjun Xiang, Congwu Ge, Xike Gao. Study on Main Chain Structure Regulation and Properties of Conjugated Copolymers Based on 2,6-Azulene and 3,4-Propylenedioxythiophene★[J]. Acta Chimica Sinica, 2023, 81(10): 1341-1349.

| Polymer | Mn a/kDa | PDIa | Td b/℃ |
|---|---|---|---|
| P(AzProDOT-1) | 11.1 | 2.29 | 370 |
| P(AzProDOT-2) | 11.4 | 2.25 | 374 |
| P(AzProDOT-3) | 9.3 | 1.46 | 394 |
| Polymer | Mn a/kDa | PDIa | Td b/℃ |
|---|---|---|---|
| P(AzProDOT-1) | 11.1 | 2.29 | 370 |
| P(AzProDOT-2) | 11.4 | 2.25 | 374 |
| P(AzProDOT-3) | 9.3 | 1.46 | 394 |
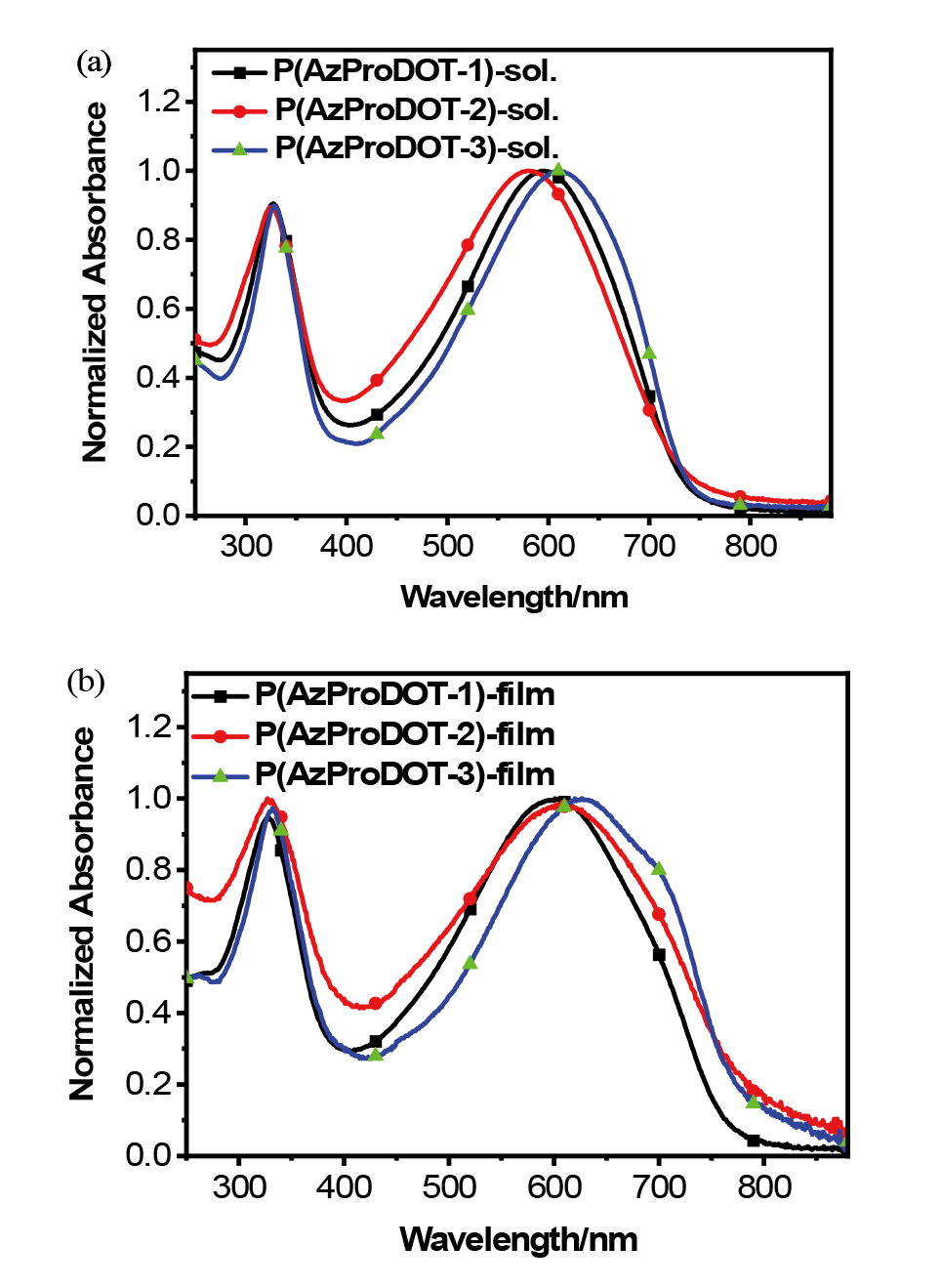
| Polymer | λmax /nm | Eopt ga/eV | ELUMO b/eV | EHOMO b/eV | ECV gc/eV | |
|---|---|---|---|---|---|---|
| Sol. | Film | |||||
| P(AzProDOT-1) | 594 | 601 | 1.60 | -3.50 | -5.36 | 1.86 |
| P(AzProDOT-2) | 587 | 603 | 1.55 | -3.48 | -5.38 | 1.90 |
| P(AzProDOT-3) | 613 | 626 | 1.58 | -3.48 | -5.40 | 1.92 |
| Polymer | λmax /nm | Eopt ga/eV | ELUMO b/eV | EHOMO b/eV | ECV gc/eV | |
|---|---|---|---|---|---|---|
| Sol. | Film | |||||
| P(AzProDOT-1) | 594 | 601 | 1.60 | -3.50 | -5.36 | 1.86 |
| P(AzProDOT-2) | 587 | 603 | 1.55 | -3.48 | -5.38 | 1.90 |
| P(AzProDOT-3) | 613 | 626 | 1.58 | -3.48 | -5.40 | 1.92 |
| [1] |
Lemal, D. M.; Goldman, G. D. J. Chem. Educ. 1988, 65, 923.
doi: 10.1021/ed065p923 |
| [2] |
(a) Koch, M.; Blacque, O.; Venkatesan, K. Org. Lett. 2012, 14, 1580.
doi: 10.1021/ol300327b |
|
(b) Tang, T.; Lin, T.; Wang, F.; He, C. J. Phys. Chem. B 2015, 119, 8176.
doi: 10.1021/acs.jpcb.5b01613 |
|
|
(c) Wang, F.; Lin, T. T.; He, C.; Chi, H.; Tang, T.; Lai, Y.-H. J. Mater. Chem. 2012, 22, 10448.
doi: 10.1039/c2jm31098h |
|
|
(d) Wang, F.; Lai, Y. H.; Kocherginsky, N. M.; Kosteski, Y. Y. Org. Lett. 2003, 5, 995.
doi: 10.1021/ol0274615 |
|
| [3] |
(a) Anderson, A. G.; Steckler, B. M. J. Am. Chem. Soc. 1959, 81, 4941.
doi: 10.1021/ja01527a046 |
|
(b) Michl, J.; Thulstrup, E. W. Tetrahedron 1976, 32, 205.
doi: 10.1016/0040-4020(76)87002-0 |
|
| [4] |
(a) Xin, H.; Li, J.; Lu, R. Q.; Gao, X.; Swager, T. M. J. Am. Chem. Soc. 2020, 142, 13598.
doi: 10.1021/jacs.0c06299 |
|
(b) Ran, H.; Duan, X.; Zheng, R.; Xie, F.; Chen, L.; Zhao, Z.; Han, R.; Lei, Z.; Hu, J. Y. ACS Appl. Mater. Interfaces 2020, 12, 23225.
doi: 10.1021/acsami.0c04552 |
|
|
(c) Yamaguchi, Y.; Maruya, Y.; Katagiri, H.; Nakayama, K.; Ohba, Y. Org. Lett. 2012, 14, 2316.
doi: 10.1021/ol3007327 |
|
|
(d) Hou, B.; Li, J.; Xin, H.; Yang, X.; Gao, H.; Peng, P.; Gao, X. Acta Chim. Sinica 2020, 78, 788 (in Chinese).
doi: 10.6023/A20050161 |
|
|
(侯斌, 李晶, 辛涵申, 杨笑迪, 高洪磊, 彭培珍, 高希珂, 化学学报, 2020, 78, 788)
|
|
|
(e) Peng, P.; Li, J.; Hou, B.; Xin, H.; Cheng, T.; Gao, X. Chin. J. Org. Chem. 2020, 40, 3916 (in Chinese).
doi: 10.6023/cjoc202005014 |
|
|
(彭培珍, 李晶, 侯斌, 辛涵申, 程探宇, 高希珂, 有机化学, 2020, 40, 3916)
|
|
| [5] |
(a) Yao, J.; Cai, Z.; Liu, Z.; Yu, C.; Luo, H.; Yang, Y.; Yang, S.; Zhang, G.; Zhang, D. Macromolecules 2015, 48, 2039.
doi: 10.1021/acs.macromol.5b00158 |
|
(b) Xin, H.; Li, J.; Ge, C.; Yang, X.; Xue, T.; Gao, X. Mater. Chem. Front. 2018, 2, 975.
doi: 10.1039/C8QM00047F |
|
|
(c) Chen, Y.; Zhu, Y.; Yang, D.; Zhao, S.; Zhang, L.; Yang, L.; Wu, J.; Huang, Y.; Xu, Z.; Lu, Z. Chemistry 2016, 22, 14527.
|
|
| [6] |
(a) Tzoganakis, N.; Feng, B.; Loizos, M.; Krassas, M.; Tsikritzis, D.; Zhuang, X.; Kymakis, E. J. Mater. Chem. C 2021, 9, 14709.
doi: 10.1039/D1TC02726C |
|
(b) Su, Y.; Li, H.; Miao, Y.; Tian, Y.; Cheng, M. Asian. J. Org. Chem. 2022, 11, e202200441.
|
|
|
(c) Zhu, W.; Zhou, K.; Fo, Y.; Li, Y.; Guo, B.; Zhang, X.; Zhou, X. Phys. Chem. Chem. Phys. 2022, 24, 18793.
doi: 10.1039/D2CP02036J |
|
| [7] |
(a) Umeyama, T.; Watanabe, Y.; Miyata, T.; Imahori, H. Chem. Lett. 2015, 44, 47.
doi: 10.1246/cl.140904 |
|
(b) Wang, X.; Ng, J. K.-P.; Jia, P.; Lin, T.; Cho, C. M.; Xu, J.; Lu, X.; He, C. Macromolecules 2009, 42, 5534.
doi: 10.1021/ma900847r |
|
|
(c) Wang, F.; Lai, Y.-H.; Han, M.-Y. Macromolecules 2004, 37, 3222.
doi: 10.1021/ma035335q |
|
|
(d) Wang, F.; Lai, Y.-H. Macromolecules 2003, 36, 536.
doi: 10.1021/ma025662i |
|
| [8] |
Murai, M.; Amir, E.; Amir, R. J.; Hawker, C. J. Chem. Sci. 2012, 3, 2721.
doi: 10.1039/c2sc20615c |
| [9] |
Xin, H.; Hou, B.; Gao, X. Acc. Chem. Res. 2021, 54, 1737.
doi: 10.1021/acs.accounts.0c00893 |
| [10] |
(a) Yamaguchi, Y.; Ogawa, K.; Nakayama, K.; Ohba, Y.; Katagiri, H. J. Am. Chem. Soc. 2013, 135, 19095.
doi: 10.1021/ja410696j |
|
(b) Yamaguchi, Y.; Takubo, M.; Ogawa, K.; Nakayama, K.; Koganezawa, T.; Katagiri, H. J. Am. Chem. Soc. 2016, 138, 11335.
doi: 10.1021/jacs.6b06877 |
|
| [11] |
Xiang, J.; Tan, W. L.; Zhang, J.; Wang, Y.; Duan, C.; McNeill, C. R.; Yang, X.; Ge, C.; Gao, X. Macromolecules 2022, 55, 8074.
doi: 10.1021/acs.macromol.2c01101 |
| [12] |
(a) Gao, H.; Ge, C.; Hou, B.; Xin, H.; Gao, X. ACS Macro Lett. 2019, 8, 1360.
doi: 10.1021/acsmacrolett.9b00657 |
|
(b) Hou, B.; Zhou, Z.; Yu, C.; Xue, X. S.; Zhang, J.; Yang, X.; Li, J.; Ge, C.; Wang, J.; Gao, X. ACS Macro Lett. 2022, 11, 680.
doi: 10.1021/acsmacrolett.2c00164 |
|
| [13] |
(a) Homyak, P.; Liu, Y.; Liu, F.; Russel, T. P.; Coughlin, E. B. Macromolecules 2015, 48, 6978.
doi: 10.1021/acs.macromol.5b01275 |
|
(b) Gobalasingham, N. S.; Pankow, R. M.; Ekiz, S.; Thompson, B. C. J. Mater. Chem. A 2017, 5, 14101.
doi: 10.1039/C7TA03980H |
|
|
(c) Broll, S.; Nübling, F.; Luzio, A.; Lentzas, D.; Komber, H.; Caironi, M.; Sommer, M. Macromolecules 2015, 48, 7481.
doi: 10.1021/acs.macromol.5b01843 |
|
|
(d) Akkuratov, A. V.; Prudnov, F. A.; Chernyak, A. V.; Kuznetsov, P. M.; Peregudov, A. S.; Troshin, P. A. J. Polym. Sci., Part A: Polym. Chem. 2019, 57, 776.
doi: 10.1002/pola.v57.7 |
|
| [14] |
(a) DiTullio, B. T.; Savagian, L. R.; Bardagot, O.; De Keersmaecker, M.; Osterholm, A. M.; Banerji, N.; Reynolds, J. R. J. Am. Chem. Soc. 2023, 145, 122.
doi: 10.1021/jacs.2c08850 |
|
(b) Mi, S.; Wu, J.; Liu, J.; Xu, Z.; Wu, X.; Luo, G.; Zheng, J.; Xu, C. ACS Appl. Mater. Interfaces 2015, 7, 27511.
doi: 10.1021/acsami.5b09717 |
|
|
(c) Luo, X.; Shen, H.; Perera, K.; Tran, D. T.; Boudouris, B. W.; Mei, J. ACS Macro Lett. 2021, 10, 1061.
doi: 10.1021/acsmacrolett.1c00328 |
|
|
(d) Yang, W.; Yue, H.-G.; Zhao, D.; Yan, H.; Cao, K.-L.; Zhao, J.-S.; Zhang, Q. Chinese J. Polym. Sci. 2021, 39, 147.
doi: 10.1007/s10118-021-2503-5 |
|
| [15] |
Meager, I.; Ashraf, R. S.; Rossbauer, S.; Bronstein, H.; Donaghey, J. E.; Marshall, J.; Schroeder, B. C.; Heeney, M.; Anthopoulos, T. D.; McCulloch, I. Macromolecules 2013, 46, 5961.
doi: 10.1021/ma401128s |
| [16] |
Fan, Q.; Martin-Jimenez, D.; Ebeling, D.; Krug, C. K.; Brechmann, L.; Kohlmeyer, C.; Hilt, G.; Hieringer, W.; Schirmeisen, A.; Gottfried, J. M. J. Am. Chem. Soc. 2019, 141, 17713.
doi: 10.1021/jacs.9b08060 |
| [17] |
Hou, B.; Li, J.; Zhou, Z. F.; Tan, W. L.; Yang, X. D.; Zhang, J. W.; McNeill, C. R.; Ge, C. W.; Wang, J. T.; Gao, X. K. ACS Mater. Lett. 2022, 4, 392.
|
| [18] |
Wang, Y.; Tan, W. L.; Xiang, J.; Ge, C.; McNeill, C. R.; Gao, X. ACS Macro Lett. 2023, 12, 487.
doi: 10.1021/acsmacrolett.3c00040 |
| [19] |
(a) Dudnik, A. S.; Aldrich, T. J.; Eastham, N. D.; Chang, R. P.; Facchetti, A.; Marks, T. J. J. Am. Chem. Soc. 2016, 138, 15699.
doi: 10.1021/jacs.6b10023 |
|
(b) Wakioka, M.; Ishiki, S.; Ozawa, F. Macromolecules 2015, 48, 8382.
doi: 10.1021/acs.macromol.5b01822 |
|
|
(c) Aldrich, T. J.; Dudnik, A. S.; Eastham, N. D.; Manley, E. F.; Chen, L. X.; Chang, R. P. H.; Melkonyan, F. S.; Facchetti, A.; Marks, T. J. Macromolecules 2018, 51, 9140.
doi: 10.1021/acs.macromol.8b02297 |
|
| [20] |
(a) Zhang, C.; Matos, T.; Li, R.; Sun, S.-S.; Lewis, J. E.; Zhang, J.; Jiang, X. Polymer Chemistry 2010, 1, 663.
doi: 10.1039/b9py00324j |
|
(b) Wang, M.; Wang, H.; Yokoyama, T.; Liu, X.; Huang, Y.; Zhang, Y.; Nguyen, T. Q.; Aramaki, S.; Bazan, G. C. J. Am. Chem. Soc. 2014, 136, 12576.
doi: 10.1021/ja506785w |
|
| [21] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery Jr, J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16, Wallingford CT, 2016.
|
| [22] |
Kim, J. S.; Fei, Z.; Wood, S.; James, D. T.; Sim, M.; Cho, K.; Heeney, M. J.; Kim, J.-S. Adv. Energy Mater. 2014, 4, 1400527.
doi: 10.1002/aenm.v4.18 |
| [23] |
Más-Montoya, M.; Janssen, R. A. J. Adv. Funct. Mater. 2017, 27, 1605779.
doi: 10.1002/adfm.v27.16 |
| [24] |
Kang, S. H.; Lee, D.; Kim, H.; Choi, W.; Oh, J.; Oh, J. H.; Yang, C. ACS Appl. Mater. Interfaces 2021, 13, 52840.
doi: 10.1021/acsami.1c14945 |
| [25] |
(a) Tu, Q.; Ma, Y.; Zhou, X.; Ma, W.; Zheng, Q. Chem. Mater. 2019, 31, 5953.
doi: 10.1021/acs.chemmater.9b02355 |
|
(b) Huang, H.; Bin, H.; Peng, Z.; Qiu, B.; Sun, C.; Liebman-Pelaez, A.; Zhang, Z.-G.; Zhu, C.; Ade, H.; Zhang, Z.; Li, Y. Macromolecules 2018, 51, 6028.
doi: 10.1021/acs.macromol.8b01036 |
|
| [26] |
Brown, A. R.; Jarrett, C. P.; de Leeuw, D. M.; Matters, M. Synth. Met. 1997, 88, 37.
doi: 10.1016/S0379-6779(97)80881-8 |
| [27] |
Tsurui, K.; Murai, M.; Ku, S.-Y.; Hawker, C. J.; Robb, M. J. Adv. Funct. Mater. 2014, 24, 7338.
doi: 10.1002/adfm.v24.46 |
| [28] |
Wang, Y.; Liu, Y.; Chen, S.; Peng, R.; Ge, Z. Chem. Mater. 2013, 25, 3196.
doi: 10.1021/cm401618h |
| [1] | 易敬霖, 陈茂. 三氟氯乙烯与甲基异丙烯基醚的光诱导共聚反应★[J]. 化学学报, 2024, 82(2): 126-131. |
| [2] | 李西安, 李孝坤. 基于温度诱导相转变共聚物和导电聚合物的自隔断超级电容器[J]. 化学学报, 2023, 81(5): 511-519. |
| [3] | 李卫华. 桥连对嵌段共聚物自组装的调控[J]. 化学学报, 2021, 79(2): 133-138. |
| [4] | 李荣烨, Khiman Mehul, 盛力, 孙静. 两亲性聚氨基酸三嵌段共聚物构筑pH/溶剂可控多级纳米结构[J]. 化学学报, 2020, 78(11): 1235-1239. |
| [5] | 崔惠娜, 邱枫, 彭娟. 一种基于聚噻吩-聚硒吩全共轭嵌段共聚物的合成及性质研究[J]. 化学学报, 2018, 76(9): 691-700. |
| [6] | 丁妍春, 俞燕蕾, 韦嘉. 不同亲疏水比例的光响应性嵌段共聚物的合成及溶液组装行为研究[J]. 化学学报, 2014, 72(5): 602-608. |
| [7] | 郝莹, 张洋, 何金林, 尚修娟, 张明祖, 倪沛红. 半乳糖胺修饰阳离子型刷形嵌段共聚物的合成与表征[J]. 化学学报, 2014, 72(5): 569-576. |
| [8] | 江昱倩, 徐海华, 赵妮, 彭谦, 帅志刚. 给受共聚物链上与链间极化子的光谱特性[J]. 化学学报, 2014, 72(2): 201-207. |
| [9] | 张广萌, 杨继萍, 陈功. 苯胺四聚体-聚乙二醇-苯胺四聚体嵌段共聚物薄膜的自组装[J]. 化学学报, 2014, 72(1): 83-88. |
| [10] | 王瑞娟, 王毅琳. 阴离子磺酸盐型Gemini表面活性剂与PEO-PPO-PEO嵌段共聚物相互作用的研究[J]. 化学学报, 2014, 72(1): 41-50. |
| [11] | 夏彬凯, 李卫华, 邱枫. 对称AB两嵌段共聚物在均聚物C中的自组装[J]. 化学学报, 2014, 72(1): 30-34. |
| [12] | 杨慧, 彭娟, 邱枫. PI-b-P2VP两嵌段共聚物环状形貌的影响因素研究[J]. 化学学报, 2013, 71(08): 1141-1148. |
| [13] | 王立权, 林嘉平, 张乾. 梳状-线性共聚物自组装的耗散粒子动力学模拟[J]. 化学学报, 2013, 71(06): 913-919. |
| [14] | 潘高翔, 冯泽, 韦嘉, 俞燕蕾. 光/温度双响应三嵌段共聚物的合成及溶液自组装行为[J]. 化学学报, 2013, 71(05): 733-738. |
| [15] | 孙暖暖, 李一鸣, 王东翔, 包木太, 童林娟. Pluronic三嵌段共聚物在油水界面上自组装行为的介观模拟研究[J]. 化学学报, 2013, 71(02): 186-192. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||