化学学报 ›› 2025, Vol. 83 ›› Issue (6): 569-578.DOI: 10.6023/A24120367 上一篇 下一篇
研究论文
杨天宇a, 代佳楠a,b, 张玉洁a, 薛娟琴a, 马晶a,*( )
)
投稿日期:2024-12-12
发布日期:2025-04-08
基金资助:
Tianyu Yanga, Jianan Daia,b, Yujie Zhanga, Juanqin Xuea, Jing Maa,*( )
)
Received:2024-12-12
Published:2025-04-08
Contact:
*E-mail: majing@xauat.edu.cn
Supported by:文章分享

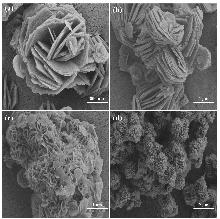
锂铝层状双氢氧化物(LiAl-LDHs)是盐湖吸附提锂离子方向中最有前途的吸附剂. 然而, 传统的LiAl-LDHs-Cl易在吸附-脱附过程中失活, 导致吸附容量低、循环稳定性差. 因此本工作提出了一种层间阴离子调控策略, 通过三种表面活性剂十二烷基硫酸钠(SDS)/聚乙烯吡咯烷酮(PVP)/柠檬酸三钠(TSC)的综合调控, 合成了新型LiAl-LDHs类材料, 并用X射线衍射(XRD)、傅里叶变换红外光谱(FT-IR)、扫描电镜(SEM)、透射电镜(TEM)、N2吸脱附等方法对吸附剂的形态结构进行了表征与分析. 结合吸附性能表明, 所合成的LiAl-LDHs-SPT呈现出更好的吸附容量(8.85 mg•g−1)、选择吸附性(Li+/Mg2+分离系数达53)和循环稳定性(可稳定使用10次以上). 在我国西台吉乃尔盐湖(吸附容量7.36 mg•g−1)与大柴旦盐湖(吸附容量8.96 mg•g−1)的实际应用中也表现优异, 解决了传统材料的缺陷. 该研究为解决传统LiAl-LDHs材料的理论缺陷提供了一种全新的改良策略, 并为盐湖吸附提锂开发提供了一种有应用前景的吸附剂.
杨天宇, 代佳楠, 张玉洁, 薛娟琴, 马晶. 层间阴离子可调控LiAl-LDHs的制备及锂吸附性能研究[J]. 化学学报, 2025, 83(6): 569-578.
Tianyu Yang, Jianan Dai, Yujie Zhang, Juanqin Xue, Jing Ma. Preparation of Interlayer Anion Controllable LiAl-LDHs and Lithium Adsorption Performance[J]. Acta Chimica Sinica, 2025, 83(6): 569-578.

| Sample | Surface area/ (m2•g−1) | Pore volume/ (cm3•g−1•nm−1) | Pore diameter/nm |
|---|---|---|---|
| LiAl-LDHs | 14.03 | 0.071 | 20.141 |
| LiAl-LDHs-ST | 15.51 | 0.107 | 8.844 |
| LiAl-LDHs-SP | 105.03 | 0.340 | 28.272 |
| LiAl-LDHs-SPT | 130.28 | 0.637 | 19.218 |
| Sample | Surface area/ (m2•g−1) | Pore volume/ (cm3•g−1•nm−1) | Pore diameter/nm |
|---|---|---|---|
| LiAl-LDHs | 14.03 | 0.071 | 20.141 |
| LiAl-LDHs-ST | 15.51 | 0.107 | 8.844 |
| LiAl-LDHs-SP | 105.03 | 0.340 | 28.272 |
| LiAl-LDHs-SPT | 130.28 | 0.637 | 19.218 |
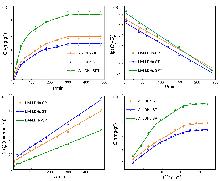

| Sample | Qe/(mg•g−1) | $Q_{e}^{*}$/(mg•g−1) | k1/min−1 | R2 |
|---|---|---|---|---|
| LiAl-LDHs-ST | 5.902 | 5.558 | 0.00418 | 0.986 |
| LiAl-LDHs-SP | 4.918 | 4.678 | 0.00427 | 0.990 |
| LiAl-LDHs-SPT | 8.852 | 7.883 | 0.00494 | 0.992 |
| Sample | Qe/(mg•g−1) | $Q_{e}^{*}$/(mg•g−1) | k1/min−1 | R2 |
|---|---|---|---|---|
| LiAl-LDHs-ST | 5.902 | 5.558 | 0.00418 | 0.986 |
| LiAl-LDHs-SP | 4.918 | 4.678 | 0.00427 | 0.990 |
| LiAl-LDHs-SPT | 8.852 | 7.883 | 0.00494 | 0.992 |
| Sample | Qe/(mg•g−1) | $Q_{e}^{*}$/(mg•g−1) | k2/(g•mg−1•min−1) | R2 |
|---|---|---|---|---|
| LiAl-LDHs-ST | 5.902 | 6.887 | 0.00188 | 0.994 |
| LiAl-LDHs-SP | 4.918 | 5.848 | 0.00206 | 0.995 |
| LiAl-LDHs-SPT | 8.852 | 9.855 | 0.00203 | 0.998 |
| Sample | Qe/(mg•g−1) | $Q_{e}^{*}$/(mg•g−1) | k2/(g•mg−1•min−1) | R2 |
|---|---|---|---|---|
| LiAl-LDHs-ST | 5.902 | 6.887 | 0.00188 | 0.994 |
| LiAl-LDHs-SP | 4.918 | 5.848 | 0.00206 | 0.995 |
| LiAl-LDHs-SPT | 8.852 | 9.855 | 0.00203 | 0.998 |
| Sample | K1a | K2a | K3a | $R_{1}^{2}$ | $R_{2}^{2}$ | $R_{3}^{2}$ |
|---|---|---|---|---|---|---|
| LiAl-LDHs-ST | 0.417 | 0.336 | 0.064 | 0.983 | 0.986 | 0.393 |
| LiAl-LDHs-SP | 0.417 | 0.250 | 0.064 | 0.983 | 0.922 | 0.393 |
| LiAl-LDHs-SPT | 0.827 | 0.379 | 0.064 | 0.986 | 0.976 | 0.393 |
| Sample | K1a | K2a | K3a | $R_{1}^{2}$ | $R_{2}^{2}$ | $R_{3}^{2}$ |
|---|---|---|---|---|---|---|
| LiAl-LDHs-ST | 0.417 | 0.336 | 0.064 | 0.983 | 0.986 | 0.393 |
| LiAl-LDHs-SP | 0.417 | 0.250 | 0.064 | 0.983 | 0.922 | 0.393 |
| LiAl-LDHs-SPT | 0.827 | 0.379 | 0.064 | 0.986 | 0.976 | 0.393 |


| Sample | Qm/(mg•g−1) | KL/min−1 | R2 |
|---|---|---|---|
| LiAl-LDHs-ST | 6.796 | 0.055 | 0.716 |
| LiAl-LDHs-SP | 5.947 | 0.045 | 0.815 |
| LiAl-LDHs-SPT | 9.281 | 0.139 | 0.850 |
| Sample | Qm/(mg•g−1) | KL/min−1 | R2 |
|---|---|---|---|
| LiAl-LDHs-ST | 6.796 | 0.055 | 0.716 |
| LiAl-LDHs-SP | 5.947 | 0.045 | 0.815 |
| LiAl-LDHs-SPT | 9.281 | 0.139 | 0.850 |
| Sample | KF/min−1 | n | R2 |
|---|---|---|---|
| LiAl-LDHs-ST | 3.352 | 13.93 | 0.914 |
| LiAl-LDHs-SP | 2.834 | 9.14 | 0.909 |
| LiAl-LDHs-SPT | 5.941 | 8.88 | 0.937 |
| Sample | KF/min−1 | n | R2 |
|---|---|---|---|
| LiAl-LDHs-ST | 3.352 | 13.93 | 0.914 |
| LiAl-LDHs-SP | 2.834 | 9.14 | 0.909 |
| LiAl-LDHs-SPT | 5.941 | 8.88 | 0.937 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
doi: 10.6023/A21070343 |
|
(赵敏, 王雪, 刘雅楠, 贺宇飞, 李殿卿, 化学学报, 2021, 79, 1518.)
doi: 10.6023/A21070343 |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
|
(王晓亮, 张多, 石雪梅, 乔心页, 程焱, 赵浩男, 常雷明, 于振秋, 黄传辉, 杨绍斌, 无机化学学报, 2023, 39, 607.)
|
|
| [29] |
|
|
(吕斌, 寇梦楠, 高党鸽, 雒康, 化工新型材料, 2023, 51, 46.)
doi: 10.19817/j.cnki.issn1006-3536.2023.04.008 |
|
| [30] |
|
|
(郑美琪, 毛方琪, 孔祥贵, 段雪, 高等学校化学学报, 2022, 43, 20220456.)
doi: 10.7503/cjcu20220456 |
|
| [31] |
|
|
(兀晓文, 杜娜, 李海平, 张人杰, 侯万国, 化学学报, 2014, 72, 963.)
doi: 10.6023/A14030146 |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
doi: 10.1016/j.jechem.2020.02.025 |
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
|
(李佳欣, 李蓓, 王纪康, 何蕾, 赵宇飞, 化学学报, 2021, 79, 238.)
doi: 10.6023/A20090441 |
|
| [41] |
|
| [42] |
|
|
(李雷, 唐鋆磊, 王丽涛, 李江涛, 全洪林, 林冰, 王宏, 李佳奇, 周太刚, 化学学报, 2024, 82, 748.)
doi: 10.6023/A24030077 |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
|
(马超, 武佳炜, 朱琳, 韩晓霞, 阮伟东, 宋薇, 王旭, 赵冰, 化学学报, 2019, 77, 1024.)
doi: 10.6023/A19050191 |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
|
(朱玉荃, 赵晓婕, 钟嫄, 陈子茹, 鄢红, 段雪, 高等学校化学学报, 2020, 41, 2287.)
doi: 10.7503/cjcu20200404 |
|
| [52] |
|
| [1] | 张蒙茜, 张玉莹, 秦家轩, 冯霄, 李雪艳, 畅通, 杨海英. 电聚合新型聚间苯二胺薄膜及H2/CO2分离性能研究[J]. 化学学报, 2025, 83(2): 132-138. |
| [2] | 谭子建, 吴腾, 乔亚军, 程瑞环, 李炜, 吴伟雄. 沸石咪唑酯骨架/正二十烷复合相变材料热导率的分子动力学模拟研究[J]. 化学学报, 2024, 82(12): 1193-1201. |
| [3] | 王海朋, 蔡文生, 邵学广. 抗冻剂抗冻机制的近红外光谱与分子模拟研究★[J]. 化学学报, 2023, 81(9): 1167-1174. |
| [4] | 王凯晴, 袁硕, 徐王东, 霍丹, 杨秋林, 侯庆喜, 于得海. ZIF-8@B-CNF复合气凝胶的制备及其吸附性能研究[J]. 化学学报, 2023, 81(6): 604-612. |
| [5] | 杨娜, 马建中, 石佳博, 郭旭. 层状复合氢氧化物的有机改性方法及应用研究进展[J]. 化学学报, 2023, 81(2): 207-216. |
| [6] | 李海茹, 张层, 李思殿. 碱土金属Ben (n=1~3)对B12团簇结构的调控研究[J]. 化学学报, 2022, 80(7): 888-895. |
| [7] | 位亚茹, 马晶, 袁婷婷, 姜嘉伟, 段银利, 薛娟琴. 氯化锂插层氮化碳材料的可控制备及吸附性能[J]. 化学学报, 2022, 80(4): 494-502. |
| [8] | 杜英喆, 张恒, 苑世领. Al2O3/PDMS复合材料热传导的分子动力学模拟[J]. 化学学报, 2021, 79(6): 787-793. |
| [9] | 徐婕, 魏雨晨, 伍智蔚, 易忠胜. 基于酸度诱导的HSA与BDE154的光谱和计算模拟研究[J]. 化学学报, 2018, 76(5): 408-414. |
| [10] | 郭宇, 姚远, 李慧, 赫兰兰, 朱尊伟, 杨忠志, 宫利东, 刘翠, 赵东霞. 光合释氧机理的ABEEM/MM/MD和BS-DFT理论研究[J]. 化学学报, 2017, 75(9): 903-913. |
| [11] | 康文斌, 夏耘, 王骏, 王炜. 二氧化硫分子通过增强二次成核促进纤维的生长:基于分子动力学的模拟研究[J]. 化学学报, 2016, 74(8): 694-702. |
| [12] | 张川, 张鲁嘉, 张洋, 黄和, 胡燚. 基于分子模拟的离子液体修饰Porcine Pancreas脂肪酶催化性能和稳定性的相关研究[J]. 化学学报, 2016, 74(1): 74-80. |
| [13] | 刘琴, 刘道彬, 何群, 项婷, Adnan Khalil, 宋礼. 基于层状过渡金属氧族化合物原位插层结构的研究进展[J]. 化学学报, 2015, 73(9): 936-943. |
| [14] | 孙婷, 刘强, 肖继军, 赵峰, 肖鹤鸣. CL-20/HMX共晶及其为基PBX界面作用和力学性能的MD模拟研究[J]. 化学学报, 2014, 72(9): 1036-1042. |
| [15] | 冯石磊, 胡墅, 刘兵, 刘伟. 抗原肽与MHC分子相互作用QM/MM分子动力学模拟研究[J]. 化学学报, 2013, 71(9): 1313-1320. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||