

季铵盐C—N键断裂构建C—X键的研究进展
收稿日期: 2021-11-27
网络出版日期: 2021-12-16
基金资助
国家自然科学基金(20672088); 国家自然科学基金(21372034); 成都市科技局(2019-YF05-02395-SN); 地质灾害防治与地质环境保护国家重点实验室(SKLGP2020Z003)
Advances in Formation of C—X Bonds via Cleavage of C—N Bond of Quaternary Ammonium Salts
Received date: 2021-11-27
Online published: 2021-12-16
Supported by
National Natural Science Foundation of China(20672088); National Natural Science Foundation of China(21372034); Chengdu Science and Technology Bureau(2019-YF05-02395-SN); State Key Laboratory of Geohazard Prevention and Geoenvironment Protection(SKLGP2020Z003)
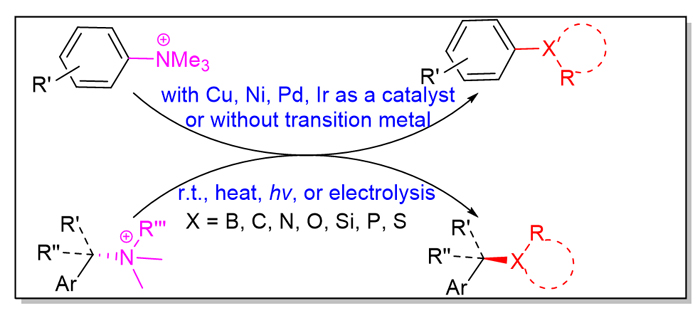
胺类种类繁多, 原料易得. 胺类的C—N键键能较大, 一般需要通过活化再进行断裂. 近些年发展了多种氨基的活化方法, 其中把胺类转化为季铵盐的活化方法, 制备容易、存放稳定, 具有一定优势. 最近十年左右, 芳香胺和苄胺衍生的季铵盐通过C—N键断裂、构建各种C—X键的研究取得了巨大的研究进展. 本综述主要论述了最近几年需要和不需要过渡金属催化的季铵盐通过C—N键断裂构建C—X键的反应. 通过C—N键断裂, 季铵盐可以构建C—B键、C—C键、C—N键、C—O键、C—Si键、C—P键、C—S键、C—Se键等, 合成硼酸酯、芳烃、烷烃、醚类、胺类、硅烷、膦、硫醚、二硫化物、硒醚、二硒化物等化合物. 而且, 如果是采用手性苄胺衍生的季铵盐, 还可以得到多种高对映体纯的手性有机化合物; 季铵盐的手性在产物中保持良好, 并且, 对所有反应都发生SN2型的构型翻转.
王一丁 , 李福海 , 曾庆乐 . 季铵盐C—N键断裂构建C—X键的研究进展[J]. 化学学报, 2022 , 80(3) : 386 -394 . DOI: 10.6023/A21110536
Amines have a wide variety and are readily available. Due to the larger C—N bond energy of amines, C—N bond of amines generally needs to be activated before cleavage. In recent years, a variety of activation methods of amino groups have been developed. Among the activation methods, converting amines into quaternary ammonium salts has certain advantages of easy preparation and stable storage. In last decade, quaternary ammonium salts derived from aromatic amines and benzylamines have made great progress in the study of constructing various C—X bonds via cleavage of C—N bond. This review mainly discusses the formation of C—X bonds of quaternary ammonium salts via cleavage of C—N bond with or without transition metal catalysts over the past few years. Via C—N bond breakage, quaternary ammonium salts can construct C—B bond, C—C bond, C—N bond, C—O bond, C—Si bond, C—P bond, C—S bond, C—Se bond, and so on. And thus borates, aromatics, alkanes, ethers, amines, silanes, phosphines, thioethers, disulfides, selenoethers, diselenides and other compounds are synthesized. Moreover, if the quaternary ammonium salts derived from chiral benzylamines are adopted, a variety of highly enantiopure chiral organic compounds are obtained. The chiralities of quaternary ammonium salts are excellently kept in the products, and SN2 type configuration reversal occurs for all of the reported reactions.

| [1] | Hartwig, J. F. Acc. Chem. Res. 1998, 31, 852. |
| [2] | Ley, S. V.; Thomas, A. W. Angew. Chem. Int. Ed. 2003, 42, 5400. |
| [3] | Schlummer, B.; Scholz, U. Adv. Synth. Catal. 2004, 346, 1599. |
| [4] | Ouyang, K.; Hao, W.; Zhang, W. X.; Xi, Z. Chem. Rev. 2015, 115, 12045. |
| [5] | Wang, Q.; Su, Y.; Li, L.; Huang, H. Chem. Soc. Rev. 2016, 45, 1257. |
| [6] | Wang, Z. X.; Yang, B. Org. Biomol. Chem. 2020, 18, 1057. |
| [7] | Li, G.; Chen, Y.; Xia, J. B. Chin. J. Org. Chem. 2018, 38, 1949. (in Chinese) |
| [7] | (李刚, 陈烨, 夏纪宝, 有机化学, 2018, 38, 1949.) |
| [8] | Song, M. M.; Zhang, Z. G.; Zheng, D.; Li, X.; Liang, R.; Zhao, X. N.; Shi, L.; Zhang, G. S. Chin. J. Org. Chem. 2020, 40, 2433. (in Chinese) |
| [8] | (宋蒙蒙, 张志国, 郑丹, 李祥, 梁蕊, 赵旭娜, 时蕾, 张贵生, 有机化学, 2020, 40, 2433.) |
| [9] | Menggen, Q.; Wu, Y.; Bao, Y. S. Chin. J. Org. Chem. 2018, 38, 902. (in Chinese) |
| [9] | (孟根其其格, 乌云, 包永胜, 有机化学, 2018, 38, 902.) |
| [10] | Zhao, Y.; Li, S. H.; Zhang, M. M.; Liu, F. Acta Chim. Sinica 2019, 77, 916. (in Chinese) |
| [10] | (赵勇, 李施宏, 张苗苗, 刘峰, 化学学报, 2019, 77, 916.) |
| [11] | Liu, J.; Yang, Y.; Ouyang, K.; Zhang, W. X. Green Synth. Catal. 2021, 2, 87. |
| [12] | Wang, C. Chem. Pharm. Bull. 2020, 68, 683. |
| [13] | Bao, H.; Qi, X.; Tambar, U. K. J. Am. Chem. Soc. 2011, 133, 1206. |
| [14] | Wenkert, E.; Han, A. L.; Jenny, C. J. J. Chem. Soc. Chem. Commnu. 1988, 975. |
| [15] | Reeves, J. T.; Fandrick, D. R.; Tan, Z.; Song, J. J.; Lee, H.; Yee, N. K.; Senanayake, C. H. Org. Lett. 2010, 12, 4388. |
| [16] | Guo, W. J.; Wang, Z. X. Tetrahedron 2013, 69, 9580. |
| [17] | Xie, L. G.; Wang, Z. X. Angew. Chem. Int. Ed. 2011, 50, 4901. |
| [18] | Ogawa, H.; Yang, Z. K.; Minami, H.; Kojima, K.; Saito, T.; Wang, C.; Uchiyama, M. ACS Catal. 2017, 7, 3988. |
| [19] | Wang, D. Y.; Kawahata, M.; Yang, Z. K.; Miyamoto, K.; Komagawa, S.; Yamaguchi, K.; Wang, C.; Uchiyama, M. Nature Commun. 2016, 7, 12937. |
| [20] | Yang, Z. K.; Wang, D. Y.; Minami, H.; Ogawa, H.; Ozaki, T.; Saito, T.; Miyamoto, K.; Wang, C.; Uchiyama, M. Chem. Eur. J. 2016, 22, 15693. |
| [21] | Blakey, S. B.; MacMillan, D. W. C. J. Am. Chem. Soc. 2003, 125, 6046. |
| [22] | Maity, P.; Shacklady-McAtee, D. M.; Yap, G. P. A.; Sirianni, E. R.; Watson, M. P. J. Am. Chem. Soc. 2013, 135, 280. |
| [23] | Chen, Q.; Gao, F.; Tang, H.; Yao, M.; Zhao, Q.; Shi, Y.; Dang, Y.; Cao, C. ACS Catal. 2019, 9, 3730. |
| [24] | Xu, S.; Zhang, Z.; Han, C.; Hu, W.; Xiao, T.; Yuan, Y.; Zhao, J. J. Org. Chem. 2019, 84, 12192. |
| [25] | Zhu, F.; Tao, J. L.; Wang, Z. X. Org. Lett. 2015, 17, 4926. |
| [26] | Han, C.; Zhang, Z.; Xu, S.; Wang, K.; Chen, K.; Zhao, J. J. Org. Chem. 2019, 84, 16308. |
| [27] | Moragas, T.; Gaydou, M.; Martin, R. Angew. Chem. Int. Ed. 2016, 55, 5053. |
| [28] | Liao, L. L.; Cao, G. M.; Ye, J. H.; Sun, G. Q.; Zhou, W. J.; Gui, Y. Y.; Yan, S. S.; Shen, G.; Yu, D. G. J. Am. Chem. Soc. 2018, 140, 17338. |
| [29] | Yu, W.; Yang, S.; Xiong, F.; Fan, T.; Feng, Y.; Huang, Y.; Fu, J.; Wang, T. Org. Biomol. Chem. 2018, 16, 3099. |
| [30] | Rand Alexander, W.; Montgomery, J. Chem. Sci. 2019, 10, 5338. |
| [31] | Scharfbier, J.; Gross, B. M.; Oestreich, M. Angew. Chem. Int. Ed. 2020, 59, 1577. |
| [32] | Zhang, X. Q.; Wang, Z. X. Org. Biomol. Chem. 2014, 12, 1448. |
| [33] | Chen, H.; Yang, H.; Li, N.; Xue, X.; He, Z.; Zeng, Q. Org. Proc. Res. Dev. 2019, 23, 1679. |
| [34] | Yang, B.; Wang, Z. X. J. Org. Chem. 2019, 84, 1500. |
| [35] | Li, N.; Chen, F.; Wang, G.; Zeng, Q. Monatsch. Chem. 2020, 151, 99. |
| [36] | O'Connor, S. E.; Grosset, A.; Janiak, P. Fund. Clin. Pharmacol. 1999, 13, 145. |
| [37] | Sobal, G.; Menzel, E. J.; Sinzinger, H. Biochem. Pharmacol. 2001, 61, 373. |
| [38] | Zeng, Q.; Wang, H.; Wang, T.; Cai, Y.; Weng, W.; Zhao, Y. Adv. Synth. Catal. 2005, 347, 1933. |
| [39] | Zhang, L.; Tan, M.; Zhou, L.; Zeng, Q. Tetrahedron Lett. 2018, 59, 2778. |
| [40] | Jiang, W.; Huang, Y.; Zhou, L.; Zeng, Q. Sci. China Chem. 2019, 62, 1213. |
| [41] | Feng, J.; Zhang, Q.; Li, F.; Yang, L.; Kuchukulla, R. R.; Zeng, Q. Synlett 2021, 32, 224. |
| [42] | Jiang, W.; Li, N.; Zhou, L.; Zeng, Q. ACS Catal. 2018, 8, 9899. |
| [43] | Chen, H.; Jiang, W.; Zeng, Q. Chem. Rec. 2020, 20, 1269. |
| [44] | Chen, H.; Zhang, Q.; Zheng, W.; Yang, H.; Zeng, Q. Asian J. Org. Chem. 2020, 9, 773. |
| [45] | Huang, Y.; Chen, H.; Zheng, W.; Zeng, Q. Tetrahedron Lett. 2020, 61, 152320. |
| [46] | Huang, Y.; Li, J.; Chen, H.; He, Z.; Zeng, Q. Chem. Rec. 2021, 21, 1216. |
| [47] | Zhang, H.; Hagihara, S.; Itami, K. Chem. Eur. J. 2015, 21, 16796. |
| [48] | Hu, J.; Sun, H.; Cai, W.; Pu, X.; Zhang, Y.; Shi, Z. J. Org. Chem. 2016, 81, 14. |
| [49] | Basch, C. H.; Cobb, K. M.; Watson, M. P. Org. Lett. 2016, 18, 136. |
| [50] | Gui, Y.; Tian, S. K. Org. Lett. 2017, 19, 1554. |
| [51] | Li, F.; Wang, D.; Chen, H.; He, Z.; Zhou, L.; Zeng, Q. Chem. Commun. 2020, 56, 13029. |
| [52] | Tang, Q.; Li, F.; Chen, F.; Yin, X.; Tang, Y.; Zeng, Q. Asian J. Org. Chem. 2021, 10, 1687. |
| [53] | Chen, F.; Li, F.; Zeng, Q. Eur. J. Org. Chem. 2021, 2021, 5605. |
| [54] | Zhang, Q.; Feng, H.; Yang, H.; He, Z.; Zeng, Q. J. Org. Chem. 2021, 86, 7806. |
| [55] | Yang, L.; Wang, B.; Yin, X.; Zeng, Q. Chem. Rec. 2021, DOI: 10.1002/tcr.202100242. |
| [56] | Mfuh, A. M.; Doyle, J. D.; Chhetri, B.; Arman, H. D.; Larionov, O. V. J. Am. Chem. Soc. 2016, 138, 2985. |
| [57] | Wang, D. Y.; Yang, Z. K.; Wang, C.; Zhang, A.; Uchiyama, M. Angew. Chem. Int. Ed. 2018, 57, 3641. |
| [58] | Wang, D. Y.; Wen, X.; Xiong, C. D.; Zhao, J. N.; Ding, C. Y.; Meng, Q.; Zhou, H.; Wang, C.; Uchiyama, M.; Lu, X. J.; Zhang, A. iScience 2019, 15, 307. |
| [59] | Yang, D. T.; Zhu, M.; Schiffer, Z. J.; Williams, K.; Song, X.; Liu, X.; Manthiram, K. ACS Catal. 2019, 9, 4699. |
/
| 〈 |
|
〉 |