

高氯酸铵热分解机理的密度泛函理论研究
收稿日期: 2023-02-28
网络出版日期: 2023-03-24
基金资助
国家自然科学基金(22175197)
Density Functional Theory Study on Thermal Decomposition Mechanisms of Ammonium Perchlorate
Received date: 2023-02-28
Online published: 2023-03-24
Supported by
National Natural Science Foundation of China(22175197)
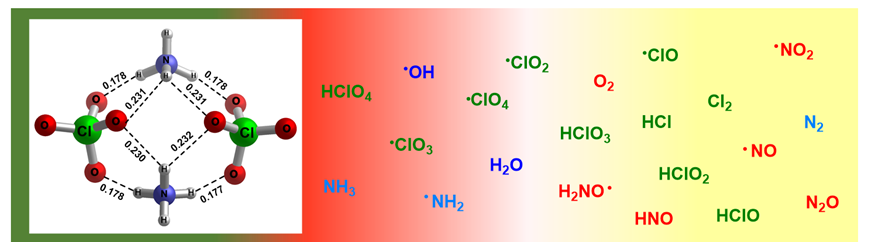
深入理解高氯酸铵的热分解机理, 对于优化固体推进剂配方设计十分重要. 我们采用对称破缺密度泛函方法 (BS-UB3LYP/6-311+G(d,p)), 对高氯酸铵的热分解机理进行了系统的梳理和深入研究. 首先, 高氯酸铵通过质子转移, 生成HClO4和NH3, 从吸附态进入气相. 进而高氯酸的Cl—OH键均裂, 生成羟基自由基•OH和三氧化氯自由基•ClO3, 它们优先和NH3反应, 生成•NH2. •NH2和HClO4反应生成•ClO4自由基, 进而和NH3反应生成H2NO, 再被自由基物种拔H生成NO. NO和•OH反应生成NO2, 和•NH2及•OH反应生成N2O. 这些产物与诸多实验观测结果一致.
杨洁 , 凌琳 , 李玉学 , 吕龙 . 高氯酸铵热分解机理的密度泛函理论研究[J]. 化学学报, 2023 , 81(4) : 328 -337 . DOI: 10.6023/A23020056
The thermal decomposition characteristics of ammonium perchlorate (AP) have a great influence on the performance of solid propellant. Although a large number of mechanistic studies have been published over the past few decades, there is no unified understanding of the decomposition yet, and the overall mechanism pathway is still unclear. In the present work, the thermal decomposition pathways of AP were studied systematically using broken-symmetry density functional theory method (BS-UB3LYP/6-311+G(d,p). This method can describe the homo-cleavage process of covalent bond well, and locate the transition state with singlet-diradical characteristics. Compared with typical multireference method, broken- symmetry density functional theory (BS-UDFT) can give good results and is much faster, and therefore is highly convenient for practical application. The results show that, the overall thermal decomposition pathway under the experimental conditions is initiated by the proton transfer between NH4+ cation and ClO4− anion, leading to neutral NH3 and HClO4 molecules, which are absorbed on the AP surface and then escape to the gas phase. The second important step is the homolytic cleavage of the Cl—OH bond in HClO4. The energy barrier is 67.5 kJ/mol under 620 K. Then, •OH radical and •ClO3 radical react with NH3 molecule, yielding •NH2 radical. Then the •NH2 radical react with HClO4, leading to •ClO4 radical, which reacts with NH3, leading to the oxidized species H2NO. The radical species, such as •OH, •NH2, •ClO and so on, abstract the H atom of H2NO, yielding NO. NO reacts with •OH radical, leading to NO2; NO reacts with •NH2 radical and •OH radical, leading to N2O. These products are consistent well with the experimental observations. Due to the complexity of the mechanisms, some strategies are used in this study: firstly, we concentrate on the reaction pathways of active species and the NH3 and HClO4 molecules, which exist in large amount; secondly, more reaction pathways involving the newly formed active species are considered.

| [1] | Thakre, P.; Yang, V. Solid Propellants, Encyclopedia of Aerospace Engineering, Eds.: Blockley, R.; Shyy, W., John Wiley & Sons, Ltd, London, 2010, pp. 1-10. |
| [2] | Mason, B. P.; Roland, C. M. Rubber Chem. Technol. 2019, 92, 1. |
| [3] | Chaturvedi, S.; Dave, P. N. Arabian. J. Chem. 2019, 12, 2061. |
| [4] | Usman, M.; Wang, L.; Yu, H.; Haq, F.; Haroon, M.; R. Summe, Ullah; Khan, A.; Fahad, S.; Nazir, A.; Elshaarani, T. J. Organomet. Chem. 2018, 872, 40. |
| [5] | Chen, T.; Hu, Y.; Zhang, C.; Gao, Z. Def. Technol. 2021, 17, 1471. |
| [6] | Miyata, K; Kubota, N. Propellants Explos. Pyrotech. 1990, 15, 127. |
| [7] | Trache, D.; Maggi, F.; Palmucci, I.; DeLuca, L. T.; Khimeche, K.; Fassina, M.; Dossi, S.; Colombo, G. Arabian. J. Chem. 2019, 12, 3639. |
| [8] | Boldyrev, V. V. Thermochim. Acta 2006, 443, 1. |
| [9] | Jacobs, P. W. M.; Whitehead, H. M. Chem. Rev. 1969, 69, 551. |
| [10] | Zhang, H.; Nie, J.; Jiao, G.; Xu, X.; Yan, S.; Guo, X.; Zhang, T. Appl. Sci. 2021, 11, 9392. |
| [11] | Heath, G. A.; Majer, J. R. Trans. Faraday Soc. 1964, 60, 1783. |
| [12] | Góbi, S.; Bergantini, A.; Turner, A. M.; Kaiser, R. I. J. Phys. Chem. A 2017, 121, 3879. |
| [13] | Mallick, L.; Kumar, S.; Chowdhury, A. Thermochim. Acta 2015, 610, 57. |
| [14] | Mallick, L.; Kumar, S.; Chowdhury, A. Thermochim. Acta 2017, 653, 83. |
| [15] | Zhu, Y.-L.; Huang, H.; Ren, H.; Jiao, Q.-J. J. Energ. Mater. 2014, 32, 16. |
| [16] | Khairetdinov, E. F.; Boldyrev, V. V. Thermochim. Acta 1980, 41, 63. |
| [17] | Bircumshaw, L. L.; Newman, B. H. Proc. R. Soc. London, Ser. A 1955, 254, 228. |
| [18] | Galwey, A. K.; Jacobs, P. W. M. Proc. R. Soc. London, Ser. A 1960, 254, 455. |
| [19] | Jacobs, P. W. M.; Russell-Jones, A. J. Phys. Chem. 1968, 72, 202. |
| [20] | Jacobs, P. W. M.; Pearson, G. S. Combust. Flame. 1969, 13, 419. |
| [21] | Liu, Z.; Yin, C.; Kong, Y.; Zhao, F.; Luo, Y.; Xiang, H. Energetic Materlals 2000, 2, 75. (in Chinese) |
| [21] | (刘子如, 阴翠梅, 孔扬辉, 赵凤起, 罗阳, 向海, 含能材料, 2000, 2, 75.) |
| [22] | Politzer, P.; Lane, P. J. Mol. Struct. THEOCHEM 1998, 454, 229. |
| [23] | Zhu, R. S.; Lin, M. C. Chem. Phys. Lett. 2006, 431, 272. |
| [24] | Zhu, R. S.; Lin, M. C. Trans. Jpn. Soc. Aeronaut. Space Sci. 2012, 10, 77. |
| [25] | Chatterjee, T.; Thynell, S. T. J. Phys. Chem. A 2021, 125, 7520. |
| [26] | Liu, M.; Liu, C.; Tsai, H. J. Chin. Chem. Soc. 2018, 65, 1437. |
| [27] | Jacobs, P. W. M.; Russell-Jones, A. AIAA J. 1967, 5, 829. |
| [28] | Levy, J. B. J. Phys. Chem. 1962, 66, 1092. |
| [29] | Fisher, I. P. Trans. Faraday Soc. 1967, 63, 684. |
| [30] | Zhou, L.; Cao, S.; Zhang, L.; Xiang, G.; Wang, J.; Zeng, X.; Chen, J. J. Hazard. Mater. 2020, 392, 122358. |
| [31] | Pearson, G. S.; Sutton, D. AIAA J. 1967, 5, 2101. |
| [32] | Zhu, R. S.; Lin, M. C. PhysChemComm 2001, 4, 127. |
| [33] | Xu, S.; Lin, M. C. Int. J. Chem. Kinet. 2009, 41, 678. |
| [34] | Lüttke, W.; Skancke, P. N.; Traetteberg, M. Theor. Chim. Acta 1994, 87, 321. |
| [35] | Ruud, K.; Helgaker, T.; Uggerud, E. J. Mol. Struct. THEOCHEM 1997, 393, 59. |
| [36] | Zhu, W.; Wei, T.; Zhu, W.; Xiao, H. J. Phys. Chem. A 2008, 112, 4688. |
| [37] | Zhu, R. S.; Lin, M. C. J. Phys. Chem. C 2008, 112, 14481. |
| [38] | For details, see supporting information SI. |
| [39] | Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, Jr., J., A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, N. J.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, ?.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2013. |
| [40] | Becke, A. D. J. Chem. Phys. 1993, 98, 5648. |
| [41] | Szabo, A.; Ostlund, N. S. Modern Quantum Chemistry: Introduction to Advanced Electronic Structure Theory, Dover, New York, 1996, pp. 221-229. |
| [42] | Grafenstein, J.; Hjerpe, A. M.; Kraka, E. J. Phys. Chem. A 2000, 104, 1748. |
| [43] | Yao, Z.; Yu, Z. J. Am. Chem. Soc. 2011, 133, 10864. |
| [44] | Ling, L.; Liu, K.; Li, X.; Li, Y. ACS Catal. 2015, 5, 2458. |
| [45] | Ling, L.; Wang, J.; Li, J.; Li, Y.; Lu, L. Chin. J. Org. Chem. 2023, 43, 285. (in Chinese) |
| [45] | (凌琳, 王健, 李婧, 李玉学, 吕龙, 有机化学, 2023, 43, 285.) |
/
| 〈 |
|
〉 |