Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (11): 1485-1493.DOI: 10.6023/A22060267 Previous Articles Next Articles
Article
王其a, 夏辉a, 熊炎威a, 张新敏a, 蔡杰a, 陈冲b, 高逸聪a, 陆峰a,*( ), 范曲立a,*(
), 范曲立a,*( )
)
投稿日期:2022-06-21
发布日期:2022-10-09
通讯作者:
陆峰, 范曲立
基金资助:
Qi Wanga, Hui Xiaa, Yanwei Xionga, Xinmin Zhanga, Jie Caia, Chong Chenb, Yicong Gaoa, Feng Lua( ), Quli Fana(
), Quli Fana( )
)
Received:2022-06-21
Published:2022-10-09
Contact:
Feng Lu, Quli Fan
Supported by:Share
Qi Wang, Hui Xia, Yanwei Xiong, Xinmin Zhang, Jie Cai, Chong Chen, Yicong Gao, Feng Lu, Quli Fan. Simple Preparation of Near-infrared-II Organic Small Molecule-based Phototheranostics by Manipulation of the Electron-donating Unit[J]. Acta Chimica Sinica, 2022, 80(11): 1485-1493.
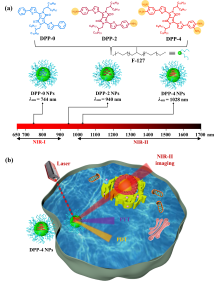





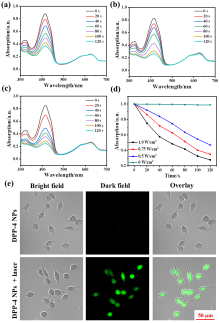



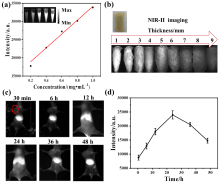



| [1] |
(a) Li, J.; Pu, K. Acc. Chem. Res. 2020, 53, 752.
doi: 10.1021/acs.accounts.9b00569 |
|
(b) Yang, K.; Yang, Z.; Yu, G.; Nie, Z.; Wang, R.; Chen, X. Adv. Mater. 2022, 34, 2107434.
|
|
| [2] |
(a) Gao, P.; Chen, Y.; Pan, W.; Li, N.; Liu, Z.; Tang, B. Angew. Chem. Int. Ed. 2021, 60, 16763.
doi: 10.1002/anie.202102574 |
|
(b) Duan, X.; Zhang, Q.; Jiang, Y.; Wu, X.; Yue, X.; Geng, Y.; Shen, J.; Ding, D. Adv. Mater. 2022, 34, 2200179.
|
|
|
(c) Xue, L.; Shen, Q.; Zhang, T.; Fan, Y. B.; Xu, X. G.; Shao, J. J.; Yang, D. L.; Zhao, W. L.; Dong, X. C.; Mou, X. Z. Mater. Chem. Front. 2021, 5, 6061.
doi: 10.1039/D1QM00631B |
|
| [3] |
(a) Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Chem. Soc. Rev. 2021, 50, 4185.
doi: 10.1039/D0CS00173B |
|
(b) Xu, Y.; Tuo, W.; Yang, L.; Sun, Y.; Li, C.; Chen, X.; Yang, W.; Yang, G.; Stang, P. J.; Sun, Y. Angew. Chem. Int. Ed. 2022, 61, 202110048.
|
|
|
(c) Yang, Z.; Zhu, Y.; Dong, Z.; Hao, Y.; Wang, C.; Li, Q.; Wu, Y.; Feng, L.; Liu, Z. Biomaterials 2022, 281, 121332.
|
|
|
(d) Li, Y.; Wang, Z.; Tang, Z. Acta Chim. Sinica 2022, 80, 291. (in Chinese)
doi: 10.6023/A21120544 |
|
|
(李嫣然, 王子贵, 汤朝晖, 化学学报, 2022, 80, 291.)
doi: 10.6023/A21120544 |
|
| [4] |
(a) Yu, Z.; Chan, W. K.; Zhang, Y.; Tan, T. T. Y. Biomaterials 2021, 269, 120459.
|
|
(b) Wang, J.; Liu, Y.; Morsch, M.; Lu, Y.; Shangguan, P.; Han, L.; Wang, Z.; Chen, X.; Song, C.; Liu, S.; Shi, B.; Tang, B. Z. Adv. Mater. 2022, 34, 2106082.
|
|
|
(c) Men, X.; Yuan, Z. ACS Appl. Polym. Mater. 2020, 2, 4319.
doi: 10.1021/acsapm.0c00715 |
|
|
(d) Xu, C.; Pu, K. Chem. Soc. Rev. 2021, 50, 1111.
doi: 10.1039/D0CS00664E |
|
|
(e) Zhang, S.; Feng, S.; Ma, L.; Yang, Y.; Liu, C.; Song, N.; Yang, Y. Acta Chim. Sinica 2022, 80, 265. (in Chinese)
doi: 10.6023/A21120548 |
|
|
(张审, 冯闪, 马陇豫, 杨莹莹, 刘超群, 宋宁宁, 杨彦伟, 化学学报, 2022, 80, 265.)
doi: 10.6023/A21120548 |
|
| [5] |
(a) Wang, Q.; Niu, X.; Yang, L.; Liu, J.; Wang, J.; Xu, X.; Tang, W.; Huang, W.; Fan, Q. Mater. Chem. Front. 2021, 5, 5689.
doi: 10.1039/D1QM00472G |
|
(b) Dai, H.; Wang, X.; Shao, J.; Wang, W.; Mou, X.; Dong, X. Small 2021, 17, 2102646.
|
|
|
(c) Sun, Y.; Zhang, Y.; Gao, Y.; Wang, P.; He, G.; Blum, N. T.; Lin, J.; Liu, Q.; Wang, X.; Huang, P. Adv. Mater. 2020, 32, 2004481.
|
|
| [6] |
(a) Sun, C.; Sun, X.; Pei, P.; He, H.; Ming, J.; Liu, X.; Liu, M.; Zhang, Y.; Xia, Y.; Zhao, D.; Li, X.; Xie, Y.; Zhang, F. Adv. Funct. Mater. 2021, 31, 2100656.
|
|
(b) Li, C.; Chen, G.; Zhang, Y.; Wu, F.; Wang, Q. J. Am. Chem. Soc. 2020, 142, 14789.
doi: 10.1021/jacs.0c07022 |
|
|
(c) Yuan, Y.; Feng, Z.; Li, S.; Huang, Z.; Wan, Y.; Cao, C.; Lin, S.; Wu, L.; Zhou, J.; Liao, L. S.; Qian, J.; Lee, C. S. Adv. Mater. 2022, 34, 2201263.
|
|
|
(d) Liu, Y.; Li, Y.; Koo, S.; Sun, Y.; Liu, Y.; Liu, X.; Pan, Y.; Zhang, Z.; Du, M.; Lu, S.; Qiao, X.; Gao, J.; Wang, X.; Deng, Z.; Meng, X.; Xiao, Y.; Kim, J. S.; Hong, X. Chem. Rev. 2022, 122, 209.
doi: 10.1021/acs.chemrev.1c00553 |
|
|
(e) Lu, F.; Sang, R.; Tang, Y.; Xia, H.; Liu, J.; Huang, W.; Fan, Q.; Wang, Q. Acta Biomater. 2022, 151, 528.
doi: 10.1016/j.actbio.2022.08.013 |
|
| [7] |
(a) Yang, H.; Huang, H.; Ma, X.; Zhang, Y.; Yang, X.; Yu, M.; Sun, Z.; Li, C.; Wu, F.; Wang, Q. Adv. Mater. 2021, 33, 2103953.
|
|
(b) Wu, D.; Liu, S.; Zhou, J.; Chen, R.; Wang, Y.; Feng, Z.; Lin, H.; Qian, J.; Tang, B. Z.; Cai, X. ACS Nano 2021, 15, 5011.
doi: 10.1021/acsnano.0c09981 |
|
|
(c) Yang, Q.; Ma, H.; Liang, Y.; Dai, H. Acc. Mater. Res. 2021, 2, 170.
doi: 10.1021/accountsmr.0c00114 |
|
|
(d) He, S.; Song, J.; Qu, J.; Cheng, Z. Chem. Soc. Rev. 2018, 47, 4258.
doi: 10.1039/C8CS00234G |
|
|
(e) Xiong, L.; Fan, Y.; Zhang, F. Acta Chim. Sinica 2019, 77, 1239. (in Chinese)
doi: 10.6023/A19080305 |
|
|
(熊麟, 凡勇, 张凡, 化学学报, 2019, 77, 1239.)
doi: 10.6023/A19080305 |
|
| [8] |
Peng, F.; Setyawati, M. I.; Tee, J. K.; Ding, X.; Wang, J.; Nga, M. E.; Ho, H. K.; Leong, D. T. Nat. Nanotechnol. 2019, 14, 279.
doi: 10.1038/s41565-018-0356-z pmid: 30692675 |
| [9] |
(a) Feng, G.; Zhang, G.; Ding, D. Chem. Soc. Rev. 2020, 49, 8179.
doi: 10.1039/D0CS00671H |
|
(b) Kenry
|
|
|
(c) Mu, J.; Xiao, M.; Shi, Y.; Geng, X.; Li, H.; Yin, Y.; Chen, X. Angew. Chem. Int. Ed. 2022, 61, 202114722.
|
|
|
(d) Sang, R.; Xu, X.; Wang, Q.; Fan, Q.; Huang, W. Acta Chim. Sinica 2020, 78, 901. (in Chinese)
doi: 10.6023/A20050190 |
|
|
(桑若愚, 许兴鹏, 王其, 范曲立, 黄维, 化学学报, 2020, 78, 901.)
doi: 10.6023/A20050190 |
|
| [10] |
Yin, C.; Tai, X.; Li, X.; Tan, J.; Lee, C.-S.; Sun, P.; Fan, Q.; Huang, W. Chem. Eng. J. 2022, 428, 132098.
|
| [11] |
Chen, J.; Wen, K.; Chen, H.; Jiang, S.; Wu, X.; Lv, L.; Peng, A.; Zhang, S.; Huang, H. Small 2020, 16, 2000909.
|
| [12] |
(a) Wang, Q.; Xu, J.; Geng, R.; Cai, J.; Li, J.; Xie, C.; Tang, W.; Shen, Q.; Huang, W.; Fan, Q. Biomaterials 2020, 231, 119671.
|
|
(b) Xu, Y.; Zhang, Y.; Li, J.; An, J.; Li, C.; Bai, S.; Sharma, A.; Deng, G.; Kim, J. S.; Sun, Y. Biomaterials 2020, 259, 120315.
|
|
|
(c) Li, Y.; Fan, X.; Li, Y.; Liu, S.; Chuah, C.; Tang, Y.; Kwok, R. T. K.; Lam, J. W. Y.; Lu, X.; Qian, J.; Tang, B. Z. ACS Nano 2022, 16, 3323.
doi: 10.1021/acsnano.1c11424 |
|
|
(d) Su, Y.; Yu, B.; Wang, S.; Cong, H.; Shen, Y. Biomaterials 2021, 271, 120717.
|
| [1] | Yuan Zhang, Beining Zheng, Meichun Fu, Shouhua Feng. Research Progress in the Application of Spinel Oxides in Tumor Therapy★ [J]. Acta Chimica Sinica, 2023, 81(8): 949-954. |
| [2] | Chen Yeqin, Chen Jinping, Yu Tianjun, Zeng Yi, Li Yi. Polysaccharide Matrix-Induced Room-Temperature Phosphorescence of Organic Small Molecules [J]. Acta Chimica Sinica, 2023, 81(5): 450-455. |
| [3] | Xin Lv, Yi Wu, Boran Zhang, Wei Guo. Design, Synthesis and Photodynamic Therapy of a H2O2-Activatable Near Infrared Borondipyrromethene (BODIPY) Photosensitizer [J]. Acta Chimica Sinica, 2023, 81(4): 359-370. |
| [4] | Yinghong Yan, Pingzhao Liang, Yang Zou, Lin Yuan, Xiaojun Peng, Jiangli Fan, Xiaobing Zhang. Structure and Properties Regulation of Organic Photosensitizers and Application in Photodiagnosis and Treatment★ [J]. Acta Chimica Sinica, 2023, 81(11): 1642-1662. |
| [5] | He Xu, Pengbo Han, Anjun Qin, Ben Zhong Tang. Recent Advances and Application Prospects in Photothermal Materials★ [J]. Acta Chimica Sinica, 2023, 81(10): 1420-1437. |
| [6] | Yanrong Jia, Guanlei Gao, Min Xia. Methods for Adjusting Mechanoluminescence Behaviors on Crystals of Purely Organic Small Molecules [J]. Acta Chimica Sinica, 2022, 80(9): 1309-1321. |
| [7] | Badi Liu, Chengjun Wang, Ying Qian. Synthesis, Two-photon Fluorescence Imaging and Photodynamic Therapy of Near Infrared Thienyl-BODIPY Photosensitizer [J]. Acta Chimica Sinica, 2022, 80(8): 1071-1083. |
| [8] | Shen Zhang, Shan Feng, Longyu Ma, Yingying Yang, Chaoqun Liu, Ningning Song, Yanwei Yang. Research of Synergistic Photothermal Antibacterial Strategy Based on Polymeric Guanidine Derivative Grafted on Mesoporous Carbon Nanospheres [J]. Acta Chimica Sinica, 2022, 80(3): 265-271. |
| [9] | Yanran Li, Zigui Wang, Zhaohui Tang. Water Soluble IR-780 Polymer for Mitochondria-Targeted Photodynamic Therapy※ [J]. Acta Chimica Sinica, 2022, 80(3): 291-296. |
| [10] | Lixiang Pan, Yanqin Huang, Kuang Sheng, Rui Zhang, Quli Fan, Wei Huang. Applications of Hyaluronic Acid Nanomaterials in Fluorescence/Photoacoustic Imaging and Phototherapy [J]. Acta Chimica Sinica, 2021, 79(9): 1097-1106. |
| [11] | Ju Huang, Zhen Li, Zhihong Liu. Functionalized Upconversion Nanoparticles for Disassembly of β‑Amyloid Aggregation with Near-Infrared Excitation [J]. Acta Chimica Sinica, 2021, 79(8): 1049-1057. |
| [12] | Sang Ruoyu, Xu Xingpeng, Wang Qi, Fan Quli, Huang Wei. Near-Infrared-II Fluorescence Probes Based on Organic Small Molecules [J]. Acta Chimica Sinica, 2020, 78(9): 901-915. |
| [13] | Yan Tao, Liu Zhenhua, Song Xinyue, Zhang Shusheng. Construction and Development of Tumor Microenvironment Stimulus-Responsive Upconversion Photodynamic Diagnosis and Treatment System [J]. Acta Chimica Sinica, 2020, 78(7): 657-669. |
| [14] | Zhang Liang, Zhao Wen-Long, Li Meng, Lu Hai-Yan, Chen Chuan-Feng. Recent Progress on Room-Temperature Phosphorescent Materials of Organic Small Molecules [J]. Acta Chimica Sinica, 2020, 78(10): 1030-1040. |
| [15] | Liu Ye, Yuan Jun, Zou Yingping, Li Yongfang. Research Progress of the FuranContaining Fused Ring Conjugated Organic Molecules and Polymers [J]. Acta Chim. Sinica, 2017, 75(3): 257-270. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||