Acta Chimica Sinica ›› 2024, Vol. 82 ›› Issue (4): 426-434.DOI: 10.6023/A24010019 Previous Articles Next Articles
Original article
宋瑞, 赵铭钦, 王帅, 卢垚, 鲍晓冰, 罗巧梅, 苟蕾, 樊小勇*( ), 李东林
), 李东林
投稿日期:2024-01-18
发布日期:2024-03-01
基金资助:
Rui Song, Mingqin Zhao, Shuai Wang, Yao Lu, Xiaobing Bao, Qiaomei Luo, Lei Gou, Xiaoyong Fan*( ), Donglin Li
), Donglin Li
Received:2024-01-18
Published:2024-03-01
Contact:
* E-mail: Supported by:Share
Rui Song, Mingqin Zhao, Shuai Wang, Yao Lu, Xiaobing Bao, Qiaomei Luo, Lei Gou, Xiaoyong Fan, Donglin Li. Three-Dimensional Porous Structure and Zincophile Gradient Enabling Dendrite Free Zinc Anode[J]. Acta Chimica Sinica, 2024, 82(4): 426-434.
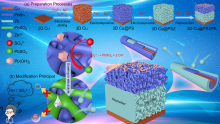



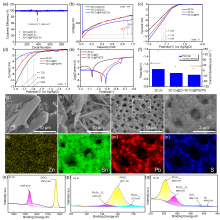



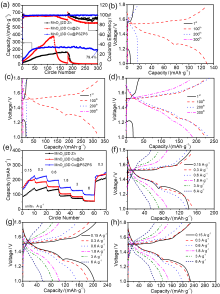

| [1] |
Zampardi, G.; La Mantia, F. Nat. Commun. 2022, 13, 687.
doi: 10.1038/s41467-022-28381-x pmid: 35115524 |
| [2] |
Li, X. Y.; Wang, L.; Fu, Y.-H.; Dang, H.; Wang, D. H.; Ran, F. Nano Energy 2023, 116, 108858.
doi: 10.1016/j.nanoen.2023.108858 |
| [3] |
Yang, J.; Yin, B.; Sun, Y.; Pan, H.; Sun, W.; Jia, B.; Zhang, S.; Ma, T. Nano-Micro Lett. 2022, 14, 42.
|
| [4] |
Wang, K. X.; Fan, X. Y.; Chen, S. J.; Deng, J. K.; Zhang, L. L.; Jing, M. S.; Li, J. L.; Gou, L.; Li, D. L.; Ma, Y. Small 2022, 19, 2206287.
doi: 10.1002/smll.v19.8 |
| [5] |
Zhao, X. Y.; Liang, X. Q.; Li, Y.; Chen, Q. G.; Chen, M. H. Energy Storage Mater. 2021, 42, 533.
|
| [6] |
Zhang, Y.; Zheng, X.; Wang, N.; Lai, W. H.; Liu, Y.; Chou, S. L.; Liu, H. K.; Dou, S. X.; Wang, Y. X. Chem. Sci. 2022, 13, 14246.
doi: 10.1039/D2SC04945G |
| [7] |
Zhou, L. F.; Du, T.; Li, J. Y.; Wang, Y. S.; Gong, H.; Yang, Q. R.; Chen, H.; Luo, W. B.; Wang, J. Z. Mater. Horiz. 2022, 9, 2722.
doi: 10.1039/d2mh00973k pmid: 36196916 |
| [8] |
Qin, R. Z.; Wang, Y. T.; Yao, L.; Yang, L. Y.; Zhao, Q. H.; Ding, S. X.; Liu, L. L.; Pan, F. Nano Energy 2022, 98, 107333.
doi: 10.1016/j.nanoen.2022.107333 |
| [9] |
Zhu, Y. Z.; Guan, P. Y.; Zhu, R. B.; Zhang, S.; Feng, Z. H.; Li, M. Y.; Wan, T.; Hu, L.; Liu, Y. J.; Li, Q.; Yu, J.; Chu, D. W. J. Energy Chem. 2023, 87, 61.
doi: 10.1016/j.jechem.2023.08.024 |
| [10] |
Zhou, M.; Guo, S.; Fang, G. Z.; Sun, H. M.; Cao, X. X.; Zhou, J.; Pan, A. Q.; Liang, S. Q. J. Energy Chem. 2021, 55, 549.
doi: 10.1016/j.jechem.2020.07.021 |
| [11] |
Wang, C.; Wang, D. D.; Lv, D.; Peng, H. L.; Song, X. X.; Yang, J.; Qian, Y. T. Adv. Energy Mater. 2023, 13, 2204388.
doi: 10.1002/aenm.v13.20 |
| [12] |
Lu, H. Y.; Liu, L. Y.; Zhang, J. K.; Xu, J. T. J. Colloid Interface Sci. 2022, 617, 422.
doi: 10.1016/j.jcis.2022.03.010 |
| [13] |
Cui, Y.; Zhao, Q.; Wu, X.; Chen, X.; Yang, J.; Wang, Y.; Qin, R.; Ding, S.; Song, Y.; Wu, J.; Yang, K.; Wang, Z.; Mei, Z.; Song, Z.; Wu, H.; Jiang, Z.; Qian, G.; Yang, L.; Pan, F. Angew. Chem., Int. Ed. 2020, 59, 16594.
doi: 10.1002/anie.v59.38 |
| [14] |
Fan, X. Y.; Yang, H.; Feng, B.; Zhu, Y. Q.; Wu, Y.; Sun, R. B.; Gou, L.; Xie, J.; Li, D. L.; Ding, Y. L. Chem. Eng. J. 2022, 445, 136799.
doi: 10.1016/j.cej.2022.136799 |
| [15] |
Ji, H. M.; Xie, C. L.; Zhang, Q.; Li, Y. X.; Li, H. H.; Wang, H. Y. Acta Chim. Sinica 2023, 81, 29. (in Chinese)
doi: 10.6023/A22100413 |
|
(姬慧敏, 谢春霖, 张旗, 李熠鑫, 李欢欢, 王海燕, 化学学报, 2023, 81, 29.)
doi: 10.6023/A22100413 |
|
| [16] |
Liu, B.; Wang, S.; Wang, Z.; Lei, H.; Chen, Z.; Mai, W. Small 2020, 16, 2001323.
doi: 10.1002/smll.v16.22 |
| [17] |
Yang, J. L.; Yang, P. H.; Yan, W. Q.; Zhao, J. W.; Fan, H. J. Energy Storage Mater. 2022, 51, 259.
|
| [18] |
Yin, Y.; Wang, S.; Zhang, Q.; Song, Y.; Chang, N.; Pan, Y.; Zhang, H.; Li, X. Adv. Mater. 2020, 32, 1906803.
doi: 10.1002/adma.v32.6 |
| [19] |
Li, Y. M.; Guo, Q.; Wu, Y.; Ying, D. F.; Yu, Y. N.; Chi, T. S.; Xia, S. J.; Zhou, X. F.; Liu, Z. P. Adv. Funct. Mater. 2023, 33, 2214523.
doi: 10.1002/adfm.v33.19 |
| [20] |
Zhao, R.; Dong, X.; Liang, P.; Li, H.; Zhang, T.; Zhou, W.; Wang, B.; Yang, Z.; Wang, X.; Wang, L.; Sun, Z.; Bu, F.; Zhao, Z.; Li, W.; Zhao, D.; Chao, D. Adv. Mater. 2023, 35, 2209288.
doi: 10.1002/adma.v35.17 |
| [21] |
Zhao, Z. M.; Lai, J.; Ho, D. T.; Lei, Y. J.; Yin, J.; Chen, L.; Schwingenschlögl, U.; Alshareef, H. N. ACS Energy Lett. 2022, 8, 608.
doi: 10.1021/acsenergylett.2c02520 |
| [22] |
Wang, Y. H.; Zhao, R.; Liu, M. Q.; Yang, J. J.; Zhang, A. Q.; Yue, J. S.; Wu, C.; Bai, Y. Adv. Energy Mater. 2023, 13, 2302707.
doi: 10.1002/aenm.v13.43 |
| [23] |
Kang, L.; Zheng, J.; Yue, K.; Yuan, H.; Luo, J.; Wang, Y.; Liu, Y.; Nai, J.; Tao, X. Small 2023, 19, 2304094.
doi: 10.1002/smll.v19.44 |
| [24] |
Shi, X.; Xie, J. H.; Wang, J.; Xie, S. L.; Yang, Z. J.; Lu, X. H. Nat. Commun. 2024, 15, 302.
doi: 10.1038/s41467-023-44615-y |
| [25] |
Shen, F.; Du, H.; Qin, H.; Wei, Z.; Kuang, W.; Hu, N.; Lv, W.; Yi, Z.; Huang, D.; Chen, Z.; He, H. Small 2023, 25, 2305119.
|
| [26] |
Zhu, K. P.; Wu, L. Y.; Guo, C.; Pu, J.; Liu, Y.; Chen, X.; Chen, Y. T.; Xue, P.; Han, J.; Yao, Y. G. Adv. Funct. Mater. 2023, 33, 2305098.
doi: 10.1002/adfm.v33.47 |
| [27] |
Gao, Y.; Cao, Q.; Pu, J.; Zhao, X.; Fu, G.; Chen, J.; Wang, Y.; Guan, C. Adv. Mater. 2023, 35, 2207573.
doi: 10.1002/adma.v35.6 |
| [28] |
Li, J.; Zou, P. C.; Chiang, S. W.; Yao, W. T.; Wang, Y.; Liu, P.; Liang, C. W.; Kang, F. Y.; Yang, C. Energy Storage Mater. 2020, 24, 700.
|
| [29] |
Cao, Q.; Gao, Y.; Pu, J.; Zhao, X.; Wang, Y.; Chen, J.; Guan, C. Nat. Commun. 2023, 14, 641.
doi: 10.1038/s41467-023-36386-3 |
| [30] |
Fan, X. Y.; Yang, H.; Wang, X. X.; Han, J. X.; Wu, Y.; Gou, L.; Li, D. L.; Ding, Y. L. Adv. Mater. Interfaces 2021, 8, 2002184.
doi: 10.1002/admi.v8.7 |
| [31] |
Liang, G. J.; Zhu, J. X.; Yan, B. X.; Li, Q.; Chen, A.; Chen, Z.; Wang, X. Q.; Xiong, B.; Fan, J.; Xu, J.; Zhi, C. Y. Energy Environ. Sci. 2022, 15, 1086.
doi: 10.1039/D1EE03749H |
| [32] |
Shen, Z. X.; Luo, L.; Li, C. W.; Pu, J.; Xie, J. P.; Wang, L. T.; Huai, Z.; Dai, Z. Y.; Yao, Y. G.; Hong, G. Adv. Energy Mater. 2021, 11, 2100214.
doi: 10.1002/aenm.v11.16 |
| [33] |
Wang, S.; Fan, X. Y.; Cui, Y.; Gou, L.; Wang, X. G.; Li, D. Acta Chim. Sinica 2019, 77, 551. (in Chinese)
doi: 10.6023/A19020057 |
|
(王珊, 樊小勇, 崔宇, 苟蕾, 王新刚, 李东林, 化学学报, 2019, 77, 551.)
doi: 10.6023/A19020057 |
|
| [34] |
Tao, F.; Liu, Y.; Ren, X. Y.; Wang, J.; Zhou, Y. Z.; Miao, Y. J.; Ren, F. Z.; Wei, S. Z.; Ma, J. M. J. Energy Chem. 2022, 66, 397.
doi: 10.1016/j.jechem.2021.08.022 |
| [35] |
Fan, X. Y.; Zhu, Y. Q.; Wu, Y.; Zhang, S.; Xu, L.; Gou, L.; Li, D. L. Chem. J. Chin. Univ. 2022, 43, 65. (in Chinese)
|
|
(樊小勇, 朱永强, 毋妍, 张帅, 许磊, 苟蕾, 李东林, 高等学校化学学报, 2022, 43, 65.)
|
|
| [36] |
Wu, Y. C.; Du, H.; Zhu, J. X.; Xu, N.; Zhou, L.; Mai, L. Q. Chem. J. Chin. Univ. 2023, 44, 61. (in Chinese)
|
|
(吴育才, 杜寰, 朱杰鑫, 许诺, 周亮, 麦立强, 高等学校化学学报, 2023, 44, 61.)
|
|
| [37] |
Xu, J.; Li, X. G.; Wang, Z. L.; Dong, C. F. Chin. J. of Nonferrous Met. 2004, 14, 1217. (in Chinese)
|
|
(徐璟, 李晓刚, 王泽力, 董超芳, 中国有色金属学报, 2004, 14, 1217.)
|
|
| [38] |
Li, H.; Guo, C.; Zhang, T.; Xue, P.; Zhao, R.; Zhou, W.; Li, W.; Elzatahry, A.; Zhao, D.; Chao, D. Nano Lett. 2022, 22, 4223.
doi: 10.1021/acs.nanolett.2c01235 |
| [39] |
Ruan, P.; Chen, X.; Qin, L.; Tang, Y.; Lu, B.; Zeng, Z.; Liang, S.; Zhou, J. Adv. Mater. 2023, 35, 2300577.
doi: 10.1002/adma.v35.31 |
| [40] |
Pan, H. L.; Shao, Y. Y.; Yan, P. F.; Cheng, Y. W.; Han, K. S.; Nie, Z. M.; Wang, C. M.; Yang, J. H.; Li, X. L.; Bhattacharya, P.; Mueller, K. T.; Liu, J. Nat. Energy 2016, 1, 16039.
doi: 10.1038/nenergy.2016.39 |
| [41] |
Fan, X. Y.; Zhang, S.; Zhu, Y. Q.; Jing, M. S.; Wang, K. X.; Zhang, L. L.; Li, J. L.; Xu, L.; Gou, L.; Li, D. L. Acta Chim. Sinica 2022, 80, 517. (in Chinese)
doi: 10.6023/A21110529 |
|
(樊小勇, 张帅, 朱永强, 敬茂森, 王凯鑫, 张露露, 李巨龙, 许磊, 苟蕾, 李东林, 化学学报, 2022, 80, 517.)
doi: 10.6023/A21110529 |
| [1] | Zaitao Hao, Jianfei Zhao, Huitong Li, Zhan Li, Lang Pan, Jiang Li. Stable Zinc Anodes through Synergistic Copper Etching and Zincophilic Polyanionic Crosslinking Membrane [J]. Acta Chimica Sinica, 2024, 82(4): 416-425. |
| [2] | Mengfan Bao, Shijie Chen, Xia Shao, Huijuan Deng, Aiqin Mao, Jie Tan. Preparation and High-rate Lithium-ion Storage of Hollow Sphere Perovskite High-entropy Oxides Assisted by Deep Eutectic Solvents [J]. Acta Chimica Sinica, 2024, 82(3): 303-313. |
| [3] | Jia Yanggang, Chen Shijie, Shao Xia, Cheng Jie, Lin Na, Fang Daolai, Mao Aiqin, Li Canhua. Preparation and High-performance Lithium-ion Storage of Cobalt-free Perovskite High-entropy Oxide Anode Materials [J]. Acta Chimica Sinica, 2023, 81(5): 486-495. |
| [4] | Yalan Zhang, Zhixiang Yuan, Hao Zhang, Jianjun Zhang, Guanglei Cui. Research Progress of High-energy-density Solid-state Lithium Ion Batteries Employing Ni-rich Ternary Cathodes [J]. Acta Chimica Sinica, 2023, 81(12): 1724-1738. |
| [5] | Wen Liu, Yujie Wang, Huiqin Yang, Chengjie Li, Na Wu, Yang Yan. The Preparation of Carbon Nanotubes/Reduced Graphene Oxide Current Collector by Non-covalent Induction of Ionic Liquid for Sodium Metal Anode [J]. Acta Chimica Sinica, 2023, 81(10): 1379-1386. |
| [6] | Guoqiang Zhang, Jinghao Huo, Xin Wang, Shouwu Guo. P-doped TiO2/C Nanotubes as Anodes for High-performance Li-ion Capacitors [J]. Acta Chimica Sinica, 2023, 81(1): 6-13. |
| [7] | Huimin Ji, Chunlin Xie, Qi Zhang, Yixin Li, Huanhuan Li, Haiyan Wang. Anode Current Collector for Aqueous Zinc-ion Batteries: Issues and Design Strategies [J]. Acta Chimica Sinica, 2023, 81(1): 29-41. |
| [8] | Shishuo Liang, Shusen Kang, Dong Yang, Jianhua Hu. Interficial Engineering of Lithium Metal Anode for Sulfide Solid State Batteries [J]. Acta Chimica Sinica, 2022, 80(9): 1264-1268. |
| [9] | Yige Wang, Hangyue Li, Zewei Lyu, Minfang Han, Kaihua Sun. Study of Operating Conditions for High Efficiency and Anode Safety of Industrial-Size Solid Oxide Fuel Cell [J]. Acta Chimica Sinica, 2022, 80(8): 1091-1099. |
| [10] | Wenchao Bi, Linfeng Zhang, Jian Chen, Ruixue Tian, Hao Huang, Man Yao. Lithiation Mechanism and Performance of Monoclinic ZnP2 Anode Materials [J]. Acta Chimica Sinica, 2022, 80(6): 756-764. |
| [11] | Xiaoyong Fan, Shuai Zhang, Yongqiang Zhu, Maosen Jing, Kaixin Wang, Lulu Zhang, Julong Li, Lei Xu, Lei Gou, Donglin Li. Construction of Dendrite-free Lithium Metal Electrode Using Three-Dimensional Porous Copper and Zinc Coatings [J]. Acta Chimica Sinica, 2022, 80(4): 517-525. |
| [12] | Rong Zhuang, Xiaosa Xu, Changzhen Qu, Shunqi Xu, Tao Yu, Hongqiang Wang, Fei Xu. Recent Progress of Porous Polymers for Lithium Metal Anodes Protection [J]. Acta Chimica Sinica, 2021, 79(4): 378-387. |
| [13] | Lu Zhang, Wenfeng Wang, Hongming Zhang, Shumin Han, Limin Wang. Research Progress and Challenge of Aqueous Zinc Ion Battery [J]. Acta Chimica Sinica, 2021, 79(2): 158-175. |
| [14] | Ruiqi Dong, Feng Wu, Ying Bai, Chuan Wu. Sodium Storage Mechanism and Optimization Strategies for Hard Carbon Anode of Sodium Ion Batteries [J]. Acta Chimica Sinica, 2021, 79(12): 1461-1476. |
| [15] | Yu Yue, Zhang Xinbo. Porous Metal-Organic Frameworks Lithium Metal Anode Protection Layer towards Long Life Li-O2 Batteries [J]. Acta Chimica Sinica, 2020, 78(12): 1434-1440. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||