Acta Chimica Sinica ›› 2024, Vol. 82 ›› Issue (4): 416-425.DOI: 10.6023/A24010006 Previous Articles Next Articles
Original article
投稿日期:2024-01-08
发布日期:2024-04-02
基金资助:
Zaitao Hao, Jianfei Zhao, Huitong Li, Zhan Li, Lang Pan, Jiang Li*( )
)
Received:2024-01-08
Published:2024-04-02
Contact:
* E-mail: Supported by:Share
Zaitao Hao, Jianfei Zhao, Huitong Li, Zhan Li, Lang Pan, Jiang Li. Stable Zinc Anodes through Synergistic Copper Etching and Zincophilic Polyanionic Crosslinking Membrane[J]. Acta Chimica Sinica, 2024, 82(4): 416-425.






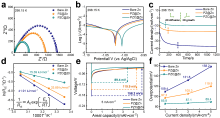

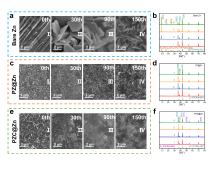





| [1] |
Chen, J. Acta Chim. Sinica 2017, 75, 127. (in Chinese)
doi: 10.6023/A1702E001 |
|
(陈军, 化学学报, 2017, 75, 127.)
|
|
| [2] |
Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K. S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; Mueller, K. T.; Liu, J. Nat. Energy. 2016, 1, 1.
doi: 10.1038/ng0492-1 |
| [3] |
Cui, M.; Xiao, Y.; Kang, L.; Du, W.; Gao, Y.; Sun, X.; Zhou, Y.; Li, X.; Li, H.; Jiang, F.; Zhi, C. ACS Appl. Energy Mater. 2019, 2, 6490.
doi: 10.1021/acsaem.9b01063 |
| [4] |
Zhang, Q.; Luan, J.; Tang, Y.; Ji, X.; Wang, H. Angew. Chem. Int. Ed. 2020, 59, 13180.
doi: 10.1002/anie.202000162 pmid: 32124537 |
| [5] |
Yang, J. L.; Li, J.; Zhao, J. W.; Liu, K.; Yang, P.; Fan, H. J. Adv. Mater. 2022, 34, 2022382.
|
| [6] |
Ji, H.-M.; Xie, C.-L.; Zhang, Q.; Li, Y.-X.; Li, H.-H.; Wang, H.-Y. Acta Chim. Sinica 2023, 81, 29. (in Chinese)
doi: 10.6023/A22100413 |
|
(姬慧敏, 谢春霖, 张旗, 李熠鑫, 李欢欢, 王海燕, 化学学报, 2023, 81, 29.)
doi: 10.6023/A22100413 |
|
| [7] |
Dong, Y.; Miao, L.; Ma, G.; Di, S.; Wang, Y.; Wang, L.; Xu, J.; Zhang, N. Chem. Sci. 2021, 12, 5843.
doi: 10.1039/D0SC06734B |
| [8] |
Song, X.; He, H.; Aboonasr Shiraz, M. H.; Zhu, H.; Khosrozadeh, A.; Liu, J. Chem. Commun. 2021, 57, 1246.
doi: 10.1039/D0CC06076C |
| [9] |
Cao, L.; Li, D.; Hu, E.; Xu, J.; Deng, T.; Ma, L.; Wang, Y.; Yang, X.-Q.; Wang, C. J. Am. Chem. Soc. 2020, 142, 21404.
doi: 10.1021/jacs.0c09794 |
| [10] |
Cao, L.; Li, D.; Pollard, T.; Deng, T.; Zhang, B.; Yang, C.; Chen, L.; Vatamanu, J.; Hu, E.; Hourwitz, M. J.; Ma, L.; Ding, M.; Li, Q.; Hou, S.; Gaskell, K.; Fourkas, J. T.; Yang, X.-Q.; Xu, K.; Borodin, O.; Wang, C. Nat. Nanotechnol. 2021, 16, 902.
doi: 10.1038/s41565-021-00905-4 |
| [11] |
Zhu, Y.; Yin, J.; Zheng, X.; Emwas, A.-H.; Lei, Y.; Mohammed, O. F.; Cui, Y.; Alshareef, H. N. Energy Environ. Sci. 2021, 14, 4463.
doi: 10.1039/D1EE01472B |
| [12] |
Han, J.; Mariani, A.; Varzi, A.; Passerini, S. J. Power Sources 2021, 485, 229329.
doi: 10.1016/j.jpowsour.2020.229329 |
| [13] |
Guo, W.; Cong, Z.; Guo, Z.; Chang, C.; Liang, X.; Liu, Y.; Hu, W.; Pu, X. Energy Storage Mater. 2020, 30, 104.
|
| [14] |
Zhou, Y.; Wang, X.; Shen, X.; Shi, Y.; Zhu, C.; Zeng, S.; Xu, H.; Cao, P.; Wang, Y.; Di, J.; Li, Q. J. Mater. Chem. A 2020, 8, 11719.
doi: 10.1039/D0TA02791J |
| [15] |
Li, S.; Fu, J.; Miao, G.; Wang, S.; Zhao, W.; Wu, Z.; Zhang, Y.; Yang, X. Adv. Mater. 2021, 33, 2008424.
doi: 10.1002/adma.v33.21 |
| [16] |
Cai, Z.; Ou, Y.; Wang, J.; Xiao, R.; Fu, L.; Yuan, Z.; Zhan, R.; Sun, Y. Energy Storage Mater. 2020, 27, 205.
|
| [17] |
Wang, Y.; Chen, Y.; Liu, W.; Ni, X.; Qing, P.; Zhao, Q.; Wei, W.; Ji, X.; Ma, J.; Chen, L. J. Mater. Chem. A 2021, 9, 8452.
doi: 10.1039/D0TA12177K |
| [18] |
Dong, L.; Yang, W.; Yang, W.; Tian, H.; Huang, Y.; Wang, X.; Xu, C.; Wang, C.; Kang, F.; Wang, G. Chem. Eng. J. 2020, 384, 123355.
doi: 10.1016/j.cej.2019.123355 |
| [19] |
Xia, A.; Pu, X.; Tao, Y.; Liu, H.; Wang, Y. Appl. Surf. Sci. 2019, 481, 852.
doi: 10.1016/j.apsusc.2019.03.197 |
| [20] |
Anand, A.; Ji Dixit, R.; Verma, A.; Basu, S. Energy Technol. 2023, 2300698.
|
| [21] |
Jiao, S.; Fu, J.; Wu, M.; Hua, T.; Hu, H. ACS Nano 2021, 16, 1013.
doi: 10.1021/acsnano.1c08638 |
| [22] |
Liu, M.-C.; Tian, C.-Y.; Zhang, D.-T.; Zhang, Y.-S.; Zhang, B.-M.; Wang, Y.-Y.; Li, C.-Y.; Liu, M.-J.; Gu, B.; Zhao, K.; Kong, L.-B.; Chueh, Y.-L. Nano Energy 2022, 103, 107805.
doi: 10.1016/j.nanoen.2022.107805 |
| [23] |
Liu, X.; Han, Q.; Ma, Q.; Wang, Y.; Liu, C. Small 2022, 18, 2203327.
doi: 10.1002/smll.v18.39 |
| [24] |
Zhao, K.; Wang, C.; Yu, Y.; Yan, M.; Wei, Q.; He, P.; Dong, Y.; Zhang, Z.; Wang, X.; Mai, L. Adv. Mater. Interfaces 2018, 5, 1800848.
|
| [25] |
Labriola, L.; Jadoul, M. Nephrol. Dial. Transplant. 2020, 35, 1455.
doi: 10.1093/ndt/gfaa004 |
| [26] |
Kwak, J. C. T.; Nelson, R. W. P. J. Phys. Chem. 1978, 82, 2388.
doi: 10.1021/j100511a008 |
| [27] |
Zhou, J.; Xie, M.; Wu, F.; Mei, Y.; Hao, Y.; Huang, R.; Wei, G.; Liu, A.; Li, L.; Chen, R. Adv. Mater. 2021, 33, 2101649.
doi: 10.1002/adma.v33.33 |
| [28] |
Zou, P.; Nykypanchuk, D.; Doerk, G.; Xin, H. L. ACS Appl. Mater. Interfaces 2021, 13, 60092.
doi: 10.1021/acsami.1c20995 |
| [29] |
Wang, H.; Chen, Y.; Yu, H.; Liu, W.; Kuang, G.; Mei, L.; Wu, Z.; Wei, W.; Ji, X.; Qu, B.; Chen, L. Adv. Funct. Mater. 2022, 32, 2205600.
doi: 10.1002/adfm.v32.43 |
| [30] |
Liang, S.; Sui, G.; Li, J.; Guo, D.; Luo, Z.; Xu, R.; Yao, H.; Wang, C.; Chen, S. Int. J. Hydrogen Energy 2022, 47, 11190.
doi: 10.1016/j.ijhydene.2022.01.154 |
| [31] |
Su, Y.; Li, S.; He, D.; Yu, D.; Liu, F.; Shao, N.; Zhang, Z. ACS Sustainable Chem. Eng. 2018, 6, 11989.
doi: 10.1021/acssuschemeng.8b02287 |
| [32] |
Fan, L.; Li, Z.; Kang, W.; Cheng, B. Renewable Energy 2020, 155, 309.
doi: 10.1016/j.renene.2020.03.153 |
| [33] |
Zhang, Q.; Luan, J.; Huang, X.; Zhu, L.; Tang, Y.; Ji, X.; Wang, H. Small 2020, 16, 2000929.
doi: 10.1002/smll.v16.35 |
| [34] |
Liang, P.; Yi, J.; Liu, X.; Wu, K.; Wang, Z.; Cui, J.; Liu, Y.; Wang, Y.; Xia, Y.; Zhang, J. Adv. Funct. Mater. 2020, 30, 1908528.
doi: 10.1002/adfm.v30.13 |
| [35] |
Lu, H.; Liu, L.; Zhang, J.; Xu, J. J. Colloid Interface Sci. 2022, 617, 422.
doi: 10.1016/j.jcis.2022.03.010 |
| [36] |
Zhao, Z.; Wang, R.; Peng, C.; Chen, W.; Wu, T.; Hu, B.; Weng, W.; Yao, Y.; Zeng, J.; Chen, Z.; Liu, P.; Liu, Y.; Li, G.; Guo, J.; Lu, H.; Guo, Z. Nat. Commun. 2021, 12, 6606.
doi: 10.1038/s41467-021-26947-9 |
| [37] |
Ma, L.; Li, Q.; Ying, Y.; Ma, F.; Chen, S.; Li, Y.; Huang, H.; Zhi, C. Adv. Mater. 2021, 33, 2007406.
doi: 10.1002/adma.v33.12 |
| [38] |
Sun, H.; Huyan, Y.; Li, N.; Lei, D.; Liu, H.; Hua, W.; Wei, C.; Kang, F.; Wang, J.-G. Nano Lett. 2023, 23, 1726.
doi: 10.1021/acs.nanolett.2c04410 |
| [39] |
Chen, T.; Wang, Y.; Yang, Y.; Huang, F.; Zhu, M.; Ang, B. T. W.; Xue, J. M. Adv. Funct. Mater. 2021, 31, 25495.
|
| [40] |
Xu, Y.; Wang, C.; Shi, Y.; Miao, G.; Fu, J.; Huang, Y. J. Mater. Chem. A 2021, 9, 25495.
doi: 10.1039/D1TA08400C |
| [41] |
Wang, Y.; Li, A.; Cheng, C. Mater. Today Chem. 2022, 26, 101057.
|
| [42] |
Li, Q.; Chen, A.; Wang, D.; Zhao, Y.; Wang, X.; Jin, X.; Xiong, B.; Zhi, C. Nat. Commun. 2022, 13, 3699.
doi: 10.1038/s41467-022-31461-7 |
| [43] |
Zhou, Y.; Xia, J.; Di, J.; Sun, Z.; Zhao, L.; Li, L.; Wu, Y.; Dong, L.; Wang, X.; Li, Q. Adv. Energy Mater. 2023, 13, 2203165.
doi: 10.1002/aenm.v13.10 |
| [44] |
Wang, Z.; Wang, Y.; Lin, Y.; Bian, G.; Liu, H.-Y.; Li, X.; Yin, J.; Zhu, J. ACS Appl. Mater. Interfaces 2022, 14, 47725.
doi: 10.1021/acsami.2c13030 |
| [1] | Wen He, Bo Wang, Hanjun Feng, Xiangru Kong, Tao Li, Rui Xiao. Research Progress of CO2 Capture and Membrane Separation by Pebax Based Materials [J]. Acta Chimica Sinica, 2024, 82(2): 226-241. |
| [2] | Xiangguo Teng, Liangwei Zhang, Xiaoyu Han, Guowei Li, Jicui Dai. Study on the Polyamine Thin Film Composite Membrane for Vanadium Battery [J]. Acta Chimica Sinica, 2024, 82(1): 16-25. |
| [3] | Shikun Liu, Chengwei Deng, Feng Ji, Yulin Min, Hexing Li. Design and Study on Pore Structure of Cathode Double Catalytic Layer in High-temperature Proton Exchange Membrane Fuel Cell★ [J]. Acta Chimica Sinica, 2023, 81(9): 1135-1141. |
| [4] | Shaojuan Zeng, Xueqi Sun, Yinge Bai, Lu Bai, Shuang Zheng, Xiangping Zhang, Suojiang Zhang. Research Progress of CO2 Capture and Separation by Functionalized Ionic Liquids and Materials★ [J]. Acta Chimica Sinica, 2023, 81(6): 627-645. |
| [5] | Huiying Zhang, Shuyan Yu, Congju Li. Electrocatalytic Degradation of Wastewater by Polymer-based Carbon Nanomembranes and Mechanism [J]. Acta Chimica Sinica, 2023, 81(4): 420-430. |
| [6] | Qingxin Wang, Yong Cui, Yunqi Li, Shanfu Lu, Yan Xiang. Effect of Controllable Pyrolysis of Ionomers in Fe-N-C Cathode Catalytic Layer on Cell Performance and Stability of Membrane Electrode Assembly★ [J]. Acta Chimica Sinica, 2023, 81(10): 1350-1356. |
| [7] | Li Sun, Yajing Wang, Tao Li, Yingshu Guo, Shusheng Zhang. Au Nanocages Probes for Mitochondrial Imaging and Photothermal Damage Cells★ [J]. Acta Chimica Sinica, 2023, 81(10): 1301-1310. |
| [8] | Min Cheng, Shihui Wang, Lei Luo, Li Zhou, Kexin Bi, Yiyang Dai, Xu Ji. Large-Scale Computational Screening of Metal-Organic Framework Membranes for Ethane/Ethylene Separation [J]. Acta Chimica Sinica, 2022, 80(9): 1277-1288. |
| [9] | Chong Li, Na Li, Limei Chang, Zhigang Gu, Jian Zhang. Research Progresses of Metal-organic Framework HKUST-1-Based Membranes in Gas Separations※ [J]. Acta Chimica Sinica, 2022, 80(3): 340-358. |
| [10] | Mengxi Zhang, Xiao Feng. Fabrication Strategies of Conjugated Microporous Polymer Membranes for Molecular Separation [J]. Acta Chimica Sinica, 2022, 80(2): 168-179. |
| [11] | Feiran Wang, Fengjing Jiang. Construction of Three-dimensional Ion-conducting Channels of Poly(vinylidene fluoride) Membranes and Their Performance in Vanadium Redox Flow Battery [J]. Acta Chimica Sinica, 2021, 79(9): 1123-1128. |
| [12] | Tengfei Yan, Shengda Liu, Yichen Luo, Yingping Zou, Junqiu Liu. Research Progress on the Macrocycle-Derived Artificial Transmembrane Ion Channels [J]. Acta Chimica Sinica, 2021, 79(8): 999-1007. |
| [13] | Luxi Lyu, Yali Zhao, Yanying Wei, Haihui Wang. Preparation of Two-Dimensional Metal-Organic Framework Membranes and Their Applications in Separation [J]. Acta Chimica Sinica, 2021, 79(7): 869-884. |
| [14] | Lili Cai, Jingyi Wang, Xuefeng Zhu, Weishen Yang. Recent Progress on Mixed Conducting Oxygen Transport Membrane Reactors for Water Splitting Reaction [J]. Acta Chimica Sinica, 2021, 79(5): 588-599. |
| [15] | Lele Li, Yangyang Xiang, Huan Liu, Shuanhong Ma, Bin Li, Zhengfeng Ma, Qiangbing Wei, Bo Yu, Feng Zhou. Temperature-Responsive Nanofibrous Membranes Fabricated by Subsurface-Initiated Atom Transfer Radical Polymerization for Controllable Oil/Water Separation [J]. Acta Chimica Sinica, 2021, 79(3): 353-360. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||