Acta Chimica Sinica ›› 2025, Vol. 83 ›› Issue (1): 17-24.DOI: 10.6023/A24100310 Previous Articles Next Articles
Original article
马金珠a, 李阳a, 高珊a, 赵越b, 丁磊c,*( ), 周东营a,*(
), 周东营a,*( ), 樊健a,*(
), 樊健a,*( )
)
投稿日期:2024-10-17
发布日期:2024-12-25
基金资助:
Jinzhu Maa, Yang Lia, Shan Gaoa, Yue Zhaob, Lei Dingc( ), Dongying Zhoua(
), Dongying Zhoua( ), Jian Fana(
), Jian Fana( )
)
Received:2024-10-17
Published:2024-12-25
Contact:
*E-mail: Supported by:Share
Jinzhu Ma, Yang Li, Shan Gao, Yue Zhao, Lei Ding, Dongying Zhou, Jian Fan. High-efficiency Near Infrared Thermally Activated Delayed Fluorescence Based on Phenazine Acceptor[J]. Acta Chimica Sinica, 2025, 83(1): 17-24.
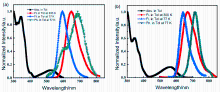
| 化合物 | λabsa/nm | λema/nm | ΦPL, tola/% | PLfl/phb/nm | (Tg/Td)c/℃ | (HOMO/LUMO)d/eV | Ege/eV | (ES1/ET1)f/eV | ΔESTg/eV | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BzPz-TPA | 346/525 | 650 | 94.0 | 595/688 | n.a./491 | -5.22/-3.13 | 2.09 | 2.08/1.80 | 0.28 | |||||||||||
| PyPz-TPA | 345/564 | 667 | 86.0 | 642/714 | 303/456 | -5.12/-3.17 | 1.95 | 1.93/1.74 | 0.19 | |||||||||||
| 化合物 | λabsa/nm | λema/nm | ΦPL, tola/% | PLfl/phb/nm | (Tg/Td)c/℃ | (HOMO/LUMO)d/eV | Ege/eV | (ES1/ET1)f/eV | ΔESTg/eV | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BzPz-TPA | 346/525 | 650 | 94.0 | 595/688 | n.a./491 | -5.22/-3.13 | 2.09 | 2.08/1.80 | 0.28 | |||||||||||
| PyPz-TPA | 345/564 | 667 | 86.0 | 642/714 | 303/456 | -5.12/-3.17 | 1.95 | 1.93/1.74 | 0.19 | |||||||||||
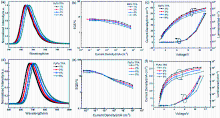
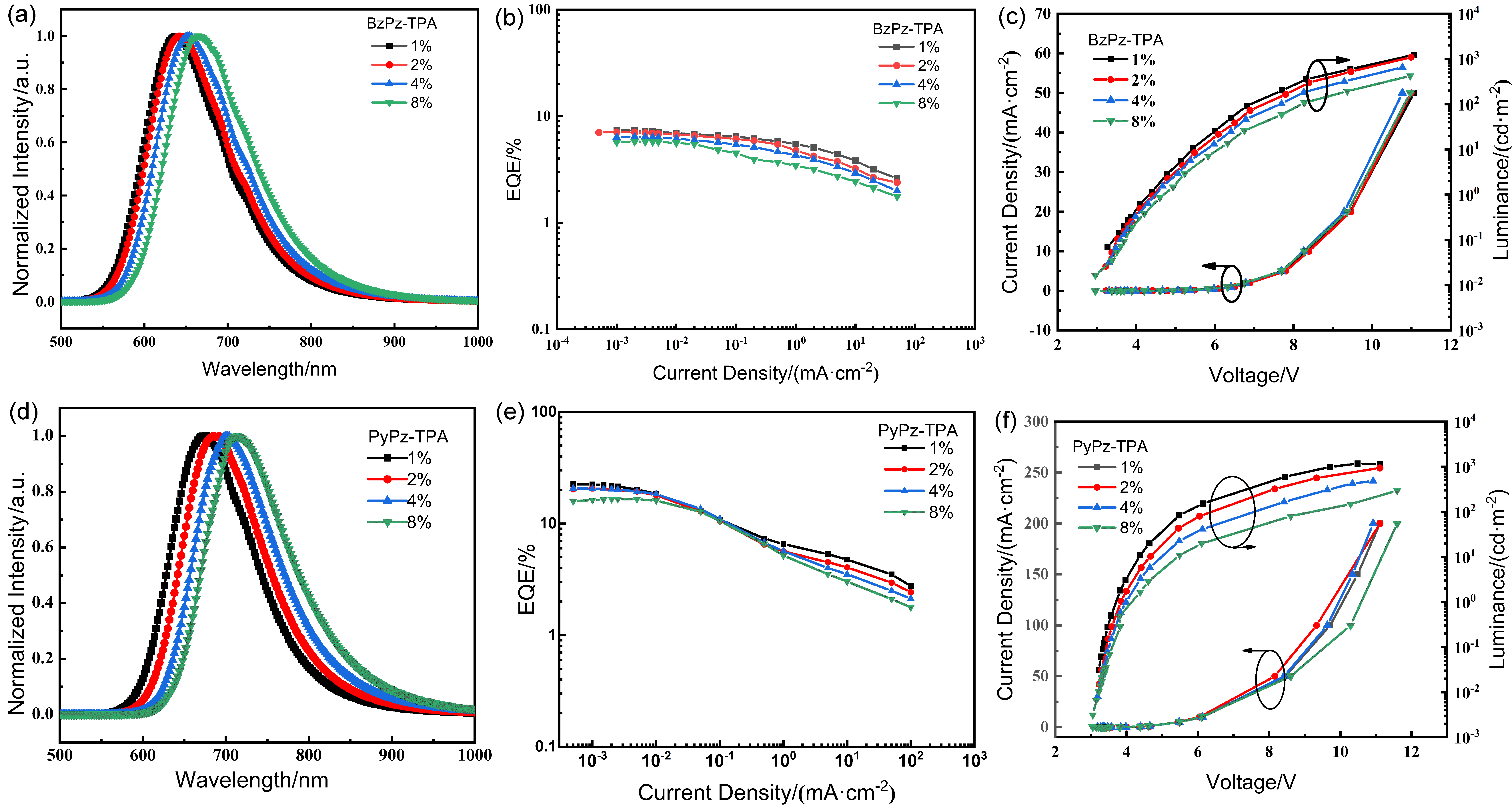
| 发光材料 | 掺杂比(w/%) | 电压a/V | CEb/(cd•A−1) | PEb/(lm•W−1) | EQEb/% | λb/nm |
|---|---|---|---|---|---|---|
| BzPz-TPA | 1 | 3.3 | 7.0 | 6.7 | 7.39 | 638 |
| 2 | 3.2 | 5.3 | 5.1 | 7.05 | 642 | |
| 4 | 3.3 | 3.2 | 3.1 | 6.33 | 654 | |
| 8 | 3.0 | 1.6 | 1.7 | 5.68 | 664 | |
| PyPz-TPA | 1 | 3.3 | 6.1 | 6.0 | 22.56 | 676 |
| 2 | 3.3 | 3.1 | 3.0 | 20.64 | 686 | |
| 4 | 3.2 | 1.6 | 1.6 | 20.74 | 700 | |
| 8 | 3.2 | 0.7 | 0.7 | 16.58 | 714 |
| 发光材料 | 掺杂比(w/%) | 电压a/V | CEb/(cd•A−1) | PEb/(lm•W−1) | EQEb/% | λb/nm |
|---|---|---|---|---|---|---|
| BzPz-TPA | 1 | 3.3 | 7.0 | 6.7 | 7.39 | 638 |
| 2 | 3.2 | 5.3 | 5.1 | 7.05 | 642 | |
| 4 | 3.3 | 3.2 | 3.1 | 6.33 | 654 | |
| 8 | 3.0 | 1.6 | 1.7 | 5.68 | 664 | |
| PyPz-TPA | 1 | 3.3 | 6.1 | 6.0 | 22.56 | 676 |
| 2 | 3.3 | 3.1 | 3.0 | 20.64 | 686 | |
| 4 | 3.2 | 1.6 | 1.6 | 20.74 | 700 | |
| 8 | 3.2 | 0.7 | 0.7 | 16.58 | 714 |
| [1] |
Tang, C. W.; VanSlyke, S. A. Appl. Phys. Lett. 1987, 51, 913.
|
| [2] |
Ge, F.-J.; Zhang, K.-Z.; Cao, Q.-P.; Xu, H.; Zhou, T.; Zhang, W.-H.; Ban, X.-X.; Zhang, X.-B.; Li, N.; Zhu, P. Acta Chim. Sinica 2023, 81, 1157 (in Chinese).
|
|
( 葛凤洁, 张开志, 曹清鹏, 徐慧, 周涛, 张文浩, 班鑫鑫, 张晓波, 李娜, 朱鹏, 化学学报, 2023, 81, 1157.)
doi: 10.6023/A23020051 |
|
| [3] |
Liu, Y. C.; Li, C. S.; Ren, Z. J.; Yan, S. K.; Bryce, M. R. Nat. Rev. Mater. 2018, 3, 18020.
|
| [4] |
Jeon, Y. M.; Choi, H. R.; Kwon, J. H.; Choi, S.; Nam, K. M.; Park, K. C.; Choi, K. C. Light Sci. Appl. 2019, 8, 114.
|
| [5] |
Zeng, X. Y.; Tang, Y. Q.; Cai, X. Y.; Tang, J. X.; Li, Y. Q. Mater. Chem. Front. 2023, 7, 1166.
|
| [6] |
Joo, W. J.; Kyoung, J.; Esfandyarpour, M.; Lee, S. H.; Koo, H.; Song, S. J.; Kwon, Y. N.; Song, S.; Bae, J. C.; Jo, A.; Kwon, M. J.; Han, S. H.; Kim, S. H.; Hwang, S. W.; Brongersma, M. L. Science 2020, 370, 459.
|
| [7] |
Zhao, S.-Y.; Zhang, X.; Lu, L.; Zhang, Y.; Zhao, Q.-H. Mater. Rep. 2020, 34, 17155 (in Chinese).
|
|
( 赵思宇, 张祥, 卢伶, 张义, 赵青华, 材料导报, 2020, 34, 17155.)
|
|
| [8] |
Wu, Z. G.; Han, H. B.; Yan, Z. P.; Luo, X. F.; Wang, Y.; Zheng, Y. X.; Zuo, J. L.; Pan, Y. Adv. Mater. 2019, 31, 1900524.
|
| [9] |
Jeon, S. K.; Lee, H. L.; Yook, K. S.; Lee, J. Y. Adv. Mater. 2019, 31, 1803524.
|
| [10] |
Wu, L.; Wang, K.; Wang, C.; Fan, X. C.; Shi, Y. Z.; Zhang, X.; Zhang, S. L.; Ye, J.; Zheng, C. J.; Li, Y. Q.; Yu, J.; Ou, X. M.; Zhang, X. H. Chem. Sci. 2021, 12, 1495.
|
| [11] |
Liu, Z.-Y.; Rao, J.-F.; Zhu, S.-J.; Wang, B.-Y.; Yu, F.; Feng, Q.-Y.; Xie, L.-H. Acta Chim. Sinica 2023, 81, 820 (in Chinese).
|
|
( 刘振宇, 饶俊峰, 祝守加, 王兵洋, 余帆, 冯全友, 解令海, 化学学报, 2023, 81, 820.)
doi: 10.6023/A23040119 |
|
| [12] |
Wei, Y. C.; Wang, S. F.; Hu, Y.; Liao, L. S.; Chen, D. G.; Chang, K. H.; Wang, C. W.; Liu, S. H.; Chan, W. H.; Liao, J. L.; Hung, W. Y.; Wang, T. H.; Chen, P. T.; Hsu, H. F.; Chi, Y.; Chou, P. T. Nat. Photonics 2020, 14, 570.
|
| [13] |
Wang, S. F.; Su, B. K.; Wang, X. Q.; Wei, Y. C.; Kuo, K. H.; Wang, C. H.; Liu, S. H.; Liao, L. S.; Hung, W. Y.; Fu, L. W.; Chuang, W. T.; Qin, M. C.; Lu, X. H.; You, C. F.; Chi, Y.; Chou, P. T. Nat. Photonics 2022, 16, 843.
|
| [14] |
Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Nature 2012, 492, 234.
|
| [15] |
Wang, S. P.; Yan, X. J.; Cheng, Z.; Zhang, H. Y.; Liu, Y.; Wang, Y. Angew. Chem. Int. Ed. 2015, 54, 13068.
|
| [16] |
Yuan, Y.; Hu, Y.; Zhang, Y. X.; Lin, J. D.; Wang, Y. K.; Jiang, Z. Q.; Liao, L. S.; Lee, S. T. Adv. Funct. Mater. 2017, 27, 1700986.
|
| [17] |
Xue, J.; Liang, Q. X.; Wang, R.; Hou, J. Y.; Li, W. Q.; Peng, Q.; Shuai, Z. G.; Qiao, J. Adv. Mater. 2019, 31, 1808242.
|
| [18] |
Congrave, D. G.; Drummond, B. H.; Conaghan, P. J.; Francis, H.; Jones, S.T.; Grey, C. P.; Greenham, N. C.; Credgington, D.; Bronstein, H. J. Am. Chem. Soc. 2019, 141, 18390.
doi: 10.1021/jacs.9b09323 pmid: 31661267 |
| [19] |
Cheng, J. F.; Pan, Z. H.; Zhang, K.; Zhao, Y.; Wang, C. K.; Ding, L.; Fung, M. K.; Fan, J. Chem. Eng. J. 2022, 430, 132744.
|
| [20] |
Wang, X.-J.; Liu, Y.-X.; Li, Y.; Yang, C.-Z.; Fung, M.-K.; Fan, J. Chem. J. Chinese Universities 2023, 44, 10 (in Chinese).
|
|
( 王霄靖, 刘艺霞, 李阳, 杨陈宗, 冯敏强, 樊健, 高等学校化学学报, 2023, 44, 10.)
|
|
| [21] |
Xu, J. Y.; Dai, Y.; Zhang, J. Y.; Jia, Z.; Meng, Q. Y.; Qiao, J. Adv. Opt. Mater. 2024, 12, 2300989.
|
| [22] |
Feng, Z. Q.; Yu, Y. J.; Song, Z. Y.; Song, M.; Zuo, P.; Jiang, Z. Q.; Zhou, D. Y.; Liao, L. S. Adv. Funct. Mater. 2024, 2403831.
|
| [23] |
Wang, Y. Y.; Zhang, Y. L.; Tong, K. N.; Ding, L.; Fan, J.; Liao, L. S. J. Mater. Chem. C 2019, 7, 15301.
|
| [24] |
He, J.-C.; Wu, J.-Q.; Wang, J.-H.; Xu, J.-W.; Tang, B.-Z.; Zhao, Z.-J. Chin. J. Org. Chem. 2024, 44, 2513 (in Chinese).
|
|
( 何俊初, 武俊琪, 王江辉, 徐静文, 唐本忠, 赵祖金, 有机化学, 2024, 44, 2513.)
doi: 10.6023/cjoc202403047 |
|
| [25] |
Yang, H.; Wang, W.-J.; Ji, J.-J.; Ding, Y.-Q.; Li, G.-H. J. Synth. Cryst. 2007, 36, 226 (in Chinese).
|
|
( 杨辉, 王维洁, 季静佳, 丁玉强, 李果华, 人工晶体学报, 2007, 36, 226.)
|
|
| [26] |
Li, Y. Y.; Li, P. C.; Lu, Z. H. Adv. Mater. 2017, 29, 1700414.
|
| [27] |
Gorse, A. D.; Pesquer, M. J. Phys. Chem. 1995, 99, 4039.
|
| [28] |
Wang, H. Q.; Zhao, B. J.; Qu, C.; Duan, C. B.; Li, Z.; Ma, P.; Chang, P.; Han, C. M.; Xu, H. Chem. Eng. J. 2022, 436, 135080.
|
| [29] |
Tai, J. W.; Tang, Y. K.; Zhang, K.; Yang, C. Z.; Pan, Z. H.; Lin, Y. C.; Shih, Y. W.; Chen, C. H.; Chiu, T. L.; Lee, J. H.; Wang, C. K.; Wu, C. C.; Fan, J. Chem. Eng. J. 2023, 452, 139534.
|
| [30] |
Zhao, T. X.; Jiang, S. S.; Wang, Y. S.; Hu, J. X.; Lin, F. L.; Meng, L. Y.; Gao, P.; Chen, X. L.; Lu, C. Z. ACS Appl. Mater. Interfaces 2023, 15, 30543.
|
| [31] |
Song, S.-F.; Zhao, D.-W.; Xu, Z.; Xu, X.-R. Chinese J. Lumin. 2006, 27, 675 (in Chinese).
|
|
( 宋淑芳, 赵德威, 徐征, 徐叙瑢, 发光学报, 2006, 27, 675.)
|
|
| [32] |
Li, Z.; Yang, D. Z.; Han, C. M.; Zhao, B. J.; Wang, H. Q.; Man, Y.; Ma, P.; Chang, P.; Ma, D. G.; Xu, H. Angew. Chem. Int. Ed. 2021, 60, 14846.
|
| [1] | Dengchao Zhang, Jihui Jia, Dong Liang, Xianbao Cai, Yuqing Zhao, Xianglong Hu, Yubing Jiang, Canzhong Lu. Design, Synthesis and Properties of Cu(I) Complexes with a Nitrogen-containing Spirocycle Ligand for Delayed Fluorescence Materials [J]. Acta Chimica Sinica, 2024, 82(8): 887-893. |
| [2] | Yuqing Zhao, Dong Liang, Jihui Jia, Rongmin Yu, Can-Zhong Lu. Synthesis and Characterization of an Emissive Ag(I) Complex with a D-A Type Ligand Containing Two Electron-withdrawing Groups [J]. Acta Chimica Sinica, 2024, 82(5): 486-492. |
| [3] | Yinuo Wang, Shiyang Shao, Lixiang Wang. Recent Advances in Multiple Resonance Organic/Polymer Fluorescent Materials with Narrowband Emission★ [J]. Acta Chimica Sinica, 2023, 81(9): 1202-1214. |
| [4] | Zhenyu Liu, Junfeng Rao, Shoujia Zhu, Bingyang Wang, Fan Yu, Quanyou Feng, Linghai Xie. Research Progress of Solution-Processed Self-Host Thermally Activated Delayed Fluorescence Materials [J]. Acta Chimica Sinica, 2023, 81(7): 820-835. |
| [5] | Yinfeng Wang, Meng Li, Chuanfeng Chen. Chiral Triptycene-Based Red Thermally Activated Delayed Fluorescence Polymers and Their Organic Light-Emitting Diodes★ [J]. Acta Chimica Sinica, 2023, 81(6): 588-594. |
| [6] | Han Zha, Jin Fang, Lingpeng Yan, Yongzhen Yang, Changqi Ma. Research Progress of Thermal Failure Mechanism and Ternary Blending to Improve Thermal Stability of Organic Solar Cells [J]. Acta Chimica Sinica, 2023, 81(2): 131-145. |
| [7] | Shaoqin Zhang, Meiqing Li, Zhongjun Zhou, Zexing Qu. Theoretical Study on the Multiple Resonance Thermally Activated Delayed Fluorescence Process [J]. Acta Chimica Sinica, 2023, 81(2): 124-130. |
| [8] | Lu Zhou, Jia-Xiong Chen, Shaomin Ji, Wen-Cheng Chen, Yanping Huo. Research Progress of Red Thermally Activated Delayed Fluorescent Materials Based on Quinoxaline [J]. Acta Chimica Sinica, 2022, 80(3): 359-372. |
| [9] | Hongtao Cao, Pengfei Hou, Qing Cao, Yanang Li, Shasha Wang, Linghai Xie. Exciplex Emission and Property Investigation Based on Cyano-substituted 9-Phenylfluorene Derivative [J]. Acta Chimica Sinica, 2022, 80(11): 1476-1484. |
| [10] | Junhui Miao, Zicheng Ding, Jun Liu, Lixiang Wang. Research Progress in Organic Solar Cells Based on Small Molecule Donors and Polymer Acceptors [J]. Acta Chimica Sinica, 2021, 79(5): 545-556. |
| [11] | Tao Zhou, Yue Qian, Hongjian Wang, Quanyou Feng, Linghai Xie, Wei Huang. Recent Advances in Substituent Effects of Blue Thermally Activated Delayed Fluorescence Small Molecules [J]. Acta Chimica Sinica, 2021, 79(5): 557-574. |
| [12] | Cao Hongtao, Li Bo, Wan Jun, Yu Tao, Xie Linghai, Sun Chen, Liu Yuyu, Wang Jin, Huang Wei. Cyano-substituted Spiro[fluorine-9,9'-xanthene] Derivatives: Exciplex Emission and Property Manipulation [J]. Acta Chimica Sinica, 2020, 78(7): 680-687. |
| [13] | Wang Zhiqiang, Bai Meidan, Zhang Ming, Zhang Zhiqiang, Feng Xun, Zheng Caijun. Synthesis and Properties of Two Novel Thermally Activated Delayed Fluorescence Materials with 1,3,5-Tribenzoylbenzene as Electron-Acceptor [J]. Acta Chimica Sinica, 2020, 78(2): 140-146. |
| [14] | Wang Zhiqiang, Cai Jialin, Zhang Ming, Zheng Caijun, Ji Baoming. A Novel Yellow Thermally Activated Delayed Fluorescence Emitter For Highly Efficient Organic Light-Emitting Diodes [J]. Acta Chim. Sinica, 2019, 77(3): 263-268. |
| [15] | Yan Zhuojun, Yuan Ye, Liu Jia, Li Qin, Nguyen Nam-Trung, Zhang Daming, Tian Yuyang, Zhu Guangshan. Targeted Syntheses of Charged Porous Aromatic Frameworks for Iodine Enrichment and Release [J]. Acta Chim. Sinica, 2016, 74(1): 67-73. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||