Acta Chimica Sinica ›› 2025, Vol. 83 ›› Issue (8): 833-843.DOI: 10.6023/A25030081 Previous Articles Next Articles
Article
成守飞a,b, 李婧c, 凌琳b, 李玉学b,*( ), 吕龙b,*(
), 吕龙b,*( )
)
投稿日期:2025-03-14
发布日期:2025-04-24
通讯作者:
李玉学, 吕龙
基金资助:
Shoufei Chenga,b, Jing Lic, Lin Lingb, Yuxue Lib,*( ), Long Lub,*(
), Long Lub,*( )
)
Received:2025-03-14
Published:2025-04-24
Contact:
Yuxue Li, Long Lu
Supported by:Share
Shoufei Cheng, Jing Li, Lin Ling, Yuxue Li, Long Lu. Density Functional Theory Research on Decomposition Mechanisms of Nitroglycerin[J]. Acta Chimica Sinica, 2025, 83(8): 833-843.
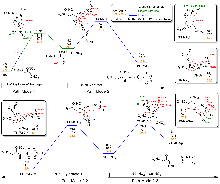

| Pathway | Gas phase | In NG | In H2O | |||||
|---|---|---|---|---|---|---|---|---|
| α | β | α | β | α | β | |||
| Path-Mode-1 | 32.8 | 33.9 | 35.9 | 35.5 | 35.0 | 33.9 | ||
| Path-Mode-2 | 61.7 | 64.0 | 63.6 | 64.4 | 58.9 | 60.3 | ||
| Path-Mode-2-2 | 65.3 | 66.1 | 62.9 | 64.4 | 61.0 | 62.0 | ||
| Path-Mode-2-3 | 67.1 | 65.8 | 67.2 | 66.2 | 64.9 | 64.4 | ||
| Path-Mode-3 | 44.9 | 45.1 | 47.0 | 47.8 | 45.7 | 46.1 | ||
| Pathway | Gas phase | In NG | In H2O | |||||
|---|---|---|---|---|---|---|---|---|
| α | β | α | β | α | β | |||
| Path-Mode-1 | 32.8 | 33.9 | 35.9 | 35.5 | 35.0 | 33.9 | ||
| Path-Mode-2 | 61.7 | 64.0 | 63.6 | 64.4 | 58.9 | 60.3 | ||
| Path-Mode-2-2 | 65.3 | 66.1 | 62.9 | 64.4 | 61.0 | 62.0 | ||
| Path-Mode-2-3 | 67.1 | 65.8 | 67.2 | 66.2 | 64.9 | 64.4 | ||
| Path-Mode-3 | 44.9 | 45.1 | 47.0 | 47.8 | 45.7 | 46.1 | ||


| Pathway | SMD in NG | SMD in H2O | |||
|---|---|---|---|---|---|
| α | β | α | β | ||
| Path-H-Mode-1 | 51.9 | 54.2 | 56.5 | 52.5 | |
| Path-H-Mode-2 | 49.3 | 50.9 | 42.1 | 43.1 | |
| Path-H-Mode-3 | 54.5 | 51.5 | 47.1 | 44.4 | |
| Path-H-Mode-4 | 39.0 | 32.5 | 29.4 | 28.1 | |
| Pathway | SMD in NG | SMD in H2O | |||
|---|---|---|---|---|---|
| α | β | α | β | ||
| Path-H-Mode-1 | 51.9 | 54.2 | 56.5 | 52.5 | |
| Path-H-Mode-2 | 49.3 | 50.9 | 42.1 | 43.1 | |
| Path-H-Mode-3 | 54.5 | 51.5 | 47.1 | 44.4 | |
| Path-H-Mode-4 | 39.0 | 32.5 | 29.4 | 28.1 | |
| M=Zn2+ | M=Mg2+ | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OH- | Cl- | OH- | Cl- | ||||||||||||||
| α | β | α | β | α | β | α | β | α | β | α | β | ||||||
| M-Mode-1 | 47.2 | 44.1 | 45.0 | 44.5 | 45.9 | 42.1 | 43.5 | 38.6 | |||||||||
| M-Mode-2 | 28.7 | 26.3 | 54.1 | 48.3 | 29.2 | 30.1 | 52.6 | 46.1 | |||||||||
| M-Mode-3 | 26.2 | 25.3 | 21.3 | 22.0 | |||||||||||||
| M-Mode-4 | 27.0 | 27.5 | 36.4 | 39.9 | 27.3 | 26.7 | 43.3 | 47.7 | |||||||||
| M=Zn2+ | M=Mg2+ | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OH- | Cl- | OH- | Cl- | ||||||||||||||
| α | β | α | β | α | β | α | β | α | β | α | β | ||||||
| M-Mode-1 | 47.2 | 44.1 | 45.0 | 44.5 | 45.9 | 42.1 | 43.5 | 38.6 | |||||||||
| M-Mode-2 | 28.7 | 26.3 | 54.1 | 48.3 | 29.2 | 30.1 | 52.6 | 46.1 | |||||||||
| M-Mode-3 | 26.2 | 25.3 | 21.3 | 22.0 | |||||||||||||
| M-Mode-4 | 27.0 | 27.5 | 36.4 | 39.9 | 27.3 | 26.7 | 43.3 | 47.7 | |||||||||
| [1] |
|
| [2] |
|
|
(乌尔班斯基著, 火炸药的化学与工艺学II, 牛秉彝, 陈绍亮译, 国防工业出版社, 北京, 1976.)
|
|
| [3] |
|
|
(托马斯•马蒂亚斯著, 张建国, 秦涧译, 高能材料化学, 北京理工大学出版社, 北京, 2016.)
|
|
| [4] |
|
|
(肯尼•范特著, 王康译, 诺贝尔全传, 世界知识出版社, 北京, 2014.)
|
|
| [5] |
|
|
(罗云军, 孙世雄, 高能固体推进剂, 北京理工大学出版社, 北京, 2023.)
|
|
| [6] |
|
| [7] |
|
|
(王振江, 薄月英, 兵工安全技术, 1996, 1, 37.)
|
|
| [8] |
|
|
(张彦才, 江业梁, 工业安全与防尘, 1990, 6, 37.)
|
|
| [9] |
|
|
(储绍华, 现代兵器, 1983, 6, 42.)
|
|
| [10] |
|
|
(张国顺, 王泽溥, 火炸药及其制品燃烧爆炸事故及其预防措施, 兵器工业出版社, 北京, 2009.)
|
|
| [11] |
|
|
(杨根, 彭松, 池旭辉, 固体火箭技术, 2009, 32, 650.)
|
|
| [12] |
|
| [13] |
|
|
(何彬, 卢先明, 孟子晖, 兵器装备工程学报, 2022, 43, 108.)
|
|
| [14] |
|
| [15] |
|
|
(姚子云, 罗秉和, 中北大学学报(自然科学版), 1989, 2, 42.)
|
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
|
(裴海潮, 吴婉娥, 付潇, 固体火箭技术, 2016, 39, 538.)
|
|
| [20] |
|
| [21] |
|
| [22] |
|
|
(裴立冠, 董可海, 唐岩辉, 含能材料, 2017, 25, 804.)
|
|
| [23] |
|
| [24] |
|
|
(丁超, 吴婉娥, 裴海潮, 化学推进剂与高分子材料, 2017, 15, 4.)
|
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
|
(刘子茹, 含能材料热分析, 国防工业出版社, 北京, 2008.)
|
|
| [29] |
|
|
(刘艳, 刘子如, 邱刚, 阴翠梅, 火炸药学报, 2001, 24, 26.)
|
|
| [30] |
|
| [31] |
|
|
(安晋川, 硝化甘油, 火炸药丛书, 1974.)
|
|
| [32] |
|
|
(方维吾, 夏正寅, 武超宇, 硝化甘油, 常规兵器工业安全技术事故资料丛书, 国防工业出版社, 北京, 1984.)
|
|
| [33] |
|
|
(曲开社, 系统工程理论与实践, 1996, 16, 82.)
|
|
| [34] |
|
| [35] |
See supporting information.
|
| [36] |
|
|
(凌琳, 王健, 李婧, 李玉学, 吕龙, 有机化学, 2023, 43, 285.)
doi: 10.6023/cjoc202206027 |
|
| [37] |
|
|
(Dean, J. A.主编, 兰氏化学手册, 尚久方译, 科学出版社, 北京, 1991.)
|
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
doi: 10.6023/A23020056 |
|
(杨洁, 凌琳, 李玉学, 吕龙, 化学学报, 2023, 81, 328.)
doi: 10.6023/A23020056 |
|
| [46] |
|
|
(崔勇康, 成守飞, 凌琳, 李玉学, 吕龙, 化学学报, 2024, 82, 377.)
doi: 10.6023/A24010017 |
|
| [47] |
|
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||