Acta Chimica Sinica ›› 2021, Vol. 79 ›› Issue (5): 678-684.DOI: 10.6023/A21010019 Previous Articles
Article
李童心1, 李东林1,*( ), 张清波1, 高建行1, 孔祥泽1, 樊小勇1, 苟蕾1
), 张清波1, 高建行1, 孔祥泽1, 樊小勇1, 苟蕾1
投稿日期:2021-01-21
发布日期:2021-03-31
通讯作者:
李东林
基金资助:
Tongxin Li1, Donglin Li1,*( ), Qingbo Zhang1, Jianhang Gao1, Xiangze Kong1, Xiaoyong Fan1, Lei Gou1
), Qingbo Zhang1, Jianhang Gao1, Xiangze Kong1, Xiaoyong Fan1, Lei Gou1
Received:2021-01-21
Published:2021-03-31
Contact:
Donglin Li
About author:Supported by:Share
Tongxin Li, Donglin Li, Qingbo Zhang, Jianhang Gao, Xiangze Kong, Xiaoyong Fan, Lei Gou. Preparation and Electrochemical Performance of Macroporous Ni-rich LiNi0.8Co0.1Mn0.1O2 Cathode Material[J]. Acta Chimica Sinica, 2021, 79(5): 678-684.
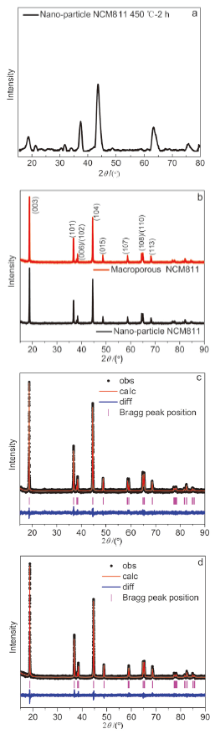
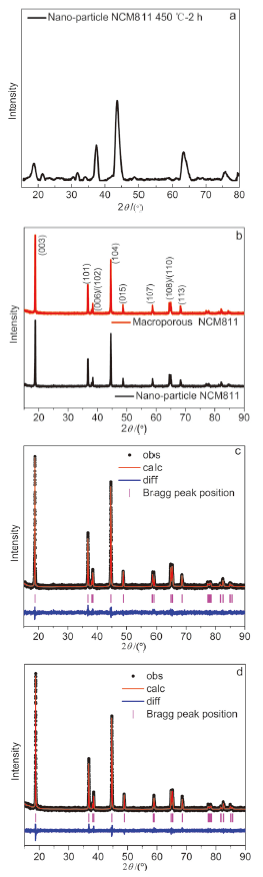
| Parameter | NCM811纳米颗粒 | 大孔NCM811 |
|---|---|---|
| Li1(3a) | 0.9468 | 0.9611 |
| Ni1(3a) | 0.0532 | 0.0389 |
| Li2(3b) | 0.0532 | 0.0389 |
| Ni2(3b) | 0.7468 | 0.7611 |
| Co1(3b) | 0.1000 | 0.1000 |
| Mn1(3b) | 0.1000 | 0.1000 |
| O1(6c) | 1.0000 | 1.0000 |
| Ni在Li位的占比/% | 5.32 | 3.89 |
| Rp/% | 6.21 | 6.25 |
| Rwp/% | 7.94 | 7.92 |
| χ2 | 1.144 | 1.130 |
| Parameter | NCM811纳米颗粒 | 大孔NCM811 |
|---|---|---|
| Li1(3a) | 0.9468 | 0.9611 |
| Ni1(3a) | 0.0532 | 0.0389 |
| Li2(3b) | 0.0532 | 0.0389 |
| Ni2(3b) | 0.7468 | 0.7611 |
| Co1(3b) | 0.1000 | 0.1000 |
| Mn1(3b) | 0.1000 | 0.1000 |
| O1(6c) | 1.0000 | 1.0000 |
| Ni在Li位的占比/% | 5.32 | 3.89 |
| Rp/% | 6.21 | 6.25 |
| Rwp/% | 7.94 | 7.92 |
| χ2 | 1.144 | 1.130 |
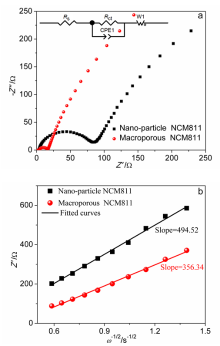
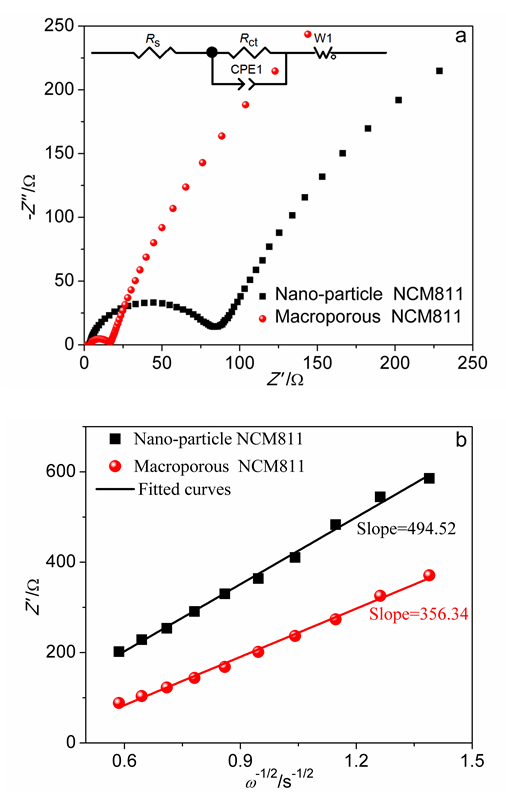
| [1] |
Lee, J.; Kitchaev, D.-A.; Kwon, D.-H.; Lee, C.-W.; Papp, J.-K.; Liu, Y.-S.; Lun, Z.-Y.; Clément, R.-J.; Shi, T.; McCloskey, B.-D.; Guo, J.-H.; Balasubramanian, M.; Ceder, G. Nature 2018, 556,185.
doi: 10.1038/s41586-018-0015-4 |
| [2] |
Qiao, Q.-Q.; Zhang, H.-Z.; Li, G.-R.; Ye, S.-H.; Wang, C.-W.; Gao, X.-P. J. Mater. Chem. A 2013, 1,5262.
doi: 10.1039/c3ta00028a |
| [3] |
Liu, J.-D.; Zhang, Y.-D.; Liu, J.-X.; Li, J.-H.; Qiu, X.-G.; Cheng, F.-Y. Acta Chim. Sinica 2020, 78,1426. (in Chinese).
doi: 10.6023/A20070330 |
|
( 刘九鼎, 张宇栋, 刘俊祥, 李金瀚, 邱晓光, 程方益, 化学学报, 2020, 78,1426.)
doi: 10.6023/A20070330 |
|
| [4] |
Li, Z.; Wang, Z.; Ban, L.-Q.; Wang, J.-T.; Lu, S.-G. Acta Chim. Sinica 2019, 77,1115. (in Chinese).
doi: 10.6023/A19070265 |
|
( 李钊, 王忠, 班丽卿, 王建涛, 卢世刚, 化学学报, 2019, 77,1115.)
doi: 10.6023/A19070265 |
|
| [5] |
Zheng, J.-C.; Yang, Z.; He, Z.-J.; Tong, H.; Yu, W.-J.; Zhang, J.-F. Nano Energy 2018, 53,613.
doi: 10.1016/j.nanoen.2018.09.014 |
| [6] |
Liu, Y.-Y.; Yang, Z.; Li, J.-L.; Niu, B.-B.; Yang, K.; Kang, F.-Y. J. Mater. Chem. A 2018, 6,13883.
doi: 10.1039/C8TA04568B |
| [7] |
Zhang, X.-D.; Hao, J.-J.; Wu, L.-C.; Guo, Z.-M.; Ji, Z.-H.; Luo, J.; Chen, C.-G.; Shu, J.-F.; Long, H.-M.; Yang, F.; Volinsky, A.-A. Electrochim. Acta 2018, 283,1203.
doi: 10.1016/j.electacta.2018.07.057 |
| [8] |
Li, Y.-C.; Zhao, W.-M.; Xiang, W.; Wu, Z.-G.; Yang, Z.-G.; Xu, C.-L.; Xu, Y.-D.; Wang, E.-H.; Wu, C.-J.; Guo, X.-D. J. Alloys Compd. 2018, 766,546.
doi: 10.1016/j.jallcom.2018.06.364 |
| [9] |
Xu, X.; Huo, H.; Jian, J.-Y.; Wang, L.-G.; Zhu, H.; Xu, S.; He, X.-S.; Yin, G.-P.; Du, C.-Y.; Sun, X.-L. Adv. Energy Mater. 2019, 9,1803963.
doi: 10.1002/aenm.v9.15 |
| [10] |
Zou, P.-J.; Lin, Z.-H.; Fan, M.-N.; Wang, F.; Liu, Y.; Xiong, X.-H. Appl. Surf. Sci. 2020, 504,144506.
doi: 10.1016/j.apsusc.2019.144506 |
| [11] |
Su, Y.-F.; Chen, G.; Chen, L.; Li, W.-K.; Zhang, Q.-Y.; Yang, Z.-R.; Lu, Y.; Bao, L.-Y.; Tan, J.; Chen, R.-J.; Chen, S.; Wu, F. ACS Appl. Mater. Interfaces 2018, 10,6407.
doi: 10.1021/acsami.7b18933 |
| [12] |
Chen, T.; Wang, F.; Li, X.; Yan, X.-X.; Wang, H.; Deng, B.-W.; Xie, Z.-W.; Qu, M.-Z. Appl. Surf. Sci. 2019, 465,863.
doi: 10.1016/j.apsusc.2018.09.250 |
| [13] |
Huang, B.; Wang, M.; Zhang, X.-W.; Zhao, Z.-Y.; Chen, L.; Gu, Y.-J. J. Alloys Compd. 2020, 830,154619.
doi: 10.1016/j.jallcom.2020.154619 |
| [14] |
Yang, H.-P.; Wu, H.-H.; Ge, M.-Y.; Li, L.-J.; Yuan, Y.-F.; Yao, Q.; Chen, J.; Xia, L.-F.; Zheng, J.-M.; Chen, Z.-Y.; Duan, J.-F.; Kisslinger, K.; Zeng, X.-C.; Lee, W.-K.; Zhang, Q.-B.; Lu, J. Adv. Funct. Mater. 2019, 29,1808825.
doi: 10.1002/adfm.v29.13 |
| [15] |
Zhu, H.-W.; Yu, H.-F.; Jiang, H.-B.; Hu, Y.-J.; Jiang, H.; Li, C.-Z. Chem. Eng. Sci. 2020, 217,115518.
doi: 10.1016/j.ces.2020.115518 |
| [16] |
Ryu, H.-H.; Park, K.-J.; Yoon, D.-R.; Aishova, A.; Yoon, C.-S.; Sun, Y.-K. Adv. Energy Mater. 2019, 9,1902698.
doi: 10.1002/aenm.v9.44 |
| [17] |
Song, B.-H.; Li, W.-D.; Oh, S.-M.; Manthiram, A. ACS Appl. Mater. Interfaces 2017, 9,9718.
doi: 10.1021/acsami.7b00070 |
| [18] |
Wang, M.; Zhang, R.; Gong, Y.-Q.; Su, Y.-F.; Xiang, D.-B.; Chen, L.; Chen, Y.-B.; Luo, M.; Chu, M. Solid State Ionics 2017, 312,53.
doi: 10.1016/j.ssi.2017.10.017 |
| [19] |
Kong, J.-Z.; Zhai, H.-F.; Ren, C.; Gao, M.-Y.; Zhang, X.; Li, H.; Li, J.-X.; Tang, Z.; Zhou, F. J. Alloys Compd. 2013, 577,507.
doi: 10.1016/j.jallcom.2013.07.007 |
| [20] |
Li, D.-L.; Tian, M.; Xie, R.; Li, Q.; Fan, X.-Y.; Gou, L.; Zhao, P.; Ma, S.-L.; Shi, Y.-X.; Yong, H.-T.-H. Nanoscale 2014, 6,3302.
doi: 10.1039/c3nr04927b |
| [21] |
Li, S.; Ma, G.; Guo, B.; Yang, Z.-H.; Fan, X.-M.; Chen, Z.-X.; Zhang, W.-X. Ind. Eng. Chem. Res. 2016, 55,9352.
doi: 10.1021/acs.iecr.6b02463 |
| [22] |
Li, L.-J.; Xu, M.; Yao, Q.; Chen, Z.-Y.; Song, L.-B.; Zhang, Z.-A.; Gao, C.-H.; Wang, P.; Yu, Z.-Y.; Lai, Y.-Q. ACS Appl. Mater. Interfaces 2016, 8,30879.
doi: 10.1021/acsami.6b09197 |
| [23] |
Ren, D.; Yang, Y.; Shen, L.-X.; Zeng, R.; Abruña, H.-D. J. Power Sources 2020, 447,227344.
doi: 10.1016/j.jpowsour.2019.227344 |
| [24] |
Su, Y.-F.; Zhang, Q.-Y.; Chen, L.; Bao, L.-Y.; Lu, Y.; Chen, S.; Wu, F. Acta Phys.-Chim. Sin. 2021, 37,1. (in Chinese).
|
|
( 苏岳锋, 张其雨, 陈来, 包丽颖, 卢赟, 陈实, 吴锋, 物理化学学报, 2021, 37,1.)
|
|
| [25] |
Gao, S.; Cheng, Y.-T.; Shirpour, M. ACS Appl. Mater. Interfaces 2019, 11,982.
doi: 10.1021/acsami.8b19349 |
| [26] |
Liu, W.; Li, X.-F.; Xiong, D.-B.; Hao, Y.-C.; Li, J.-W.; Kou, H.-R.; Yan, B.; Li, D.-J.; Lu, S.-G.; Koo, A.; Adair, K.; Sun, X.-L. Nano Energy 2018, 44,111.
doi: 10.1016/j.nanoen.2017.11.010 |
| [27] |
Tang, Z.-H.; Zheng, H.-H.; Qian, F.-P.; Ma, Y.-H.; Zhao, C.-Y.; Song, L.-B.; Chen, Y.; Xiong, X.; Zhu, X.-X.; Mi, C. Ionics 2018, 24,61.
doi: 10.1007/s11581-017-2179-6 |
| [28] |
Luo, D.; Li, G.-S.; Fu, C.-C.; Zheng, J.; Fan, J.-M.; Li, Q.; Li, L.-P. Adv. Energy Mater. 2014, 4,1400062.
doi: 10.1002/aenm.201400062 |
| [29] |
Wang, H.; Ge, W.-J.; Li, W.; Wang, F.; Liu, W.-J.; Qu, M.-Z.; Peng, G.-C. ACS Appl. Mater. Interfaces 2016, 8,18439.
doi: 10.1021/acsami.6b04644 |
| [30] |
Aishova, A.; Park, G.-T.; Yoon, C.-S.; Sun, Y.-K. Adv. Energy Mater. 2020, 10,1903179.
doi: 10.1002/aenm.v10.4 |
| [31] |
Song, X.; Liu, G.-X.; Yue, H.-F.; Luo, L.; Yang, S.-Y.; Huang, Y.-Y.; Wang, C.-R. Chem. Eng. J. 2021, 407,126301.
doi: 10.1016/j.cej.2020.126301 |
| [32] |
Zheng, Z.; Wu, Z.-G.; Xiang, W.; Guo, X.-D. Acta Chim. Sinica 2017, 75,501. (in Chinese).
doi: 10.6023/A16110594 |
|
( 郑卓, 吴振国, 向伟, 郭孝东, 化学学报, 2017, 75,501.)
doi: 10.6023/A16110594 |
|
| [33] |
Zhu, X.-J.; Chen, H.-H.; Zhan, H.; Liu, H.-X.; Yang, D.-L.; Zhou, Y.-H. Chin. J. Chem. 2005, 23,491.
doi: 10.1002/(ISSN)1614-7065 |
| [34] |
Song, J.-W.; Kim, J.-Y.; Kang, T.-W.; Kim, D.-C. Sci. Rep. 2017, 7,42521.
doi: 10.1038/srep42521 |
| [35] |
Ho, V.-C.; Jeong, S.-H.; Yim, T.; Mun, J.-Y. J. Power Sources 2020, 450,227625.
doi: 10.1016/j.jpowsour.2019.227625 |
| [36] |
Ding, G.-Y.; Gao, Y.; Li, Y.-H.; Zhu, Z.; Wang, Q.-L.; Jing, X.-G.; Yan, F.-Q.; Xu, G.-J.; Yue, Z.-H.; Li, X.-M.; Sun, F.-G. Chin. J. Inorg. Chem. 2020, 36,2307. (in Chinese).
|
|
( 丁国彧, 高远, 李亚辉, 朱振, 王秋琳, 景鑫国, 严奉乾, 徐国军, 岳之浩, 李晓敏, 孙福根, 无机化学学报, 2020, 36,2307.)
|
|
| [37] |
He, Y.-L.; Li, Y.; Liu, Y.; Li, W.-X.; Liu, W.-B. Mater. Chem. Phys. 2020, 251,123085.
doi: 10.1016/j.matchemphys.2020.123085 |
| [38] |
Yao, W.-L.; Liu, Y.; Li, D.; Zhang, Q.; Zhong, S.-W.; Cheng, H.-W.; Yan, Z.-Q. J. Phys. Chem. C 2020, 124,2346.
doi: 10.1021/acs.jpcc.9b10526 |
| [39] |
Sim, S.-J.; Lee, S.-H.; Jin, B.-S.; Kim, H.-S. Sci. Rep. 2019, 9,8952.
doi: 10.1038/s41598-019-45556-7 |
| [40] |
Wu, Z.-Z.; Ji, S.-P.; Liu, T.-C.; Duan, Y.-D.; Xiao, S.; Lin, Y.; Xu, K.; Pan, F. Nano Lett. 2016, 16,6357.
doi: 10.1021/acs.nanolett.6b02742 |
| [41] |
Zhu, W.; Tai, Z.-G.; Shu, C.-Y.; Chong, S.-K.; Guo, S.-W.; Ji, L.-J.; Chen, Y.-Z.; Liu, Y.-N. J. Mater. Chem. A 2020, 8,7991.
doi: 10.1039/D0TA00355G |
| [42] |
Li, L.-J.; Chen, Z.-Y.; Zhang, Q.-B.; Xu, M.; Zhou, X.; Zhu, H.-L.; Zhang, K.-L. J. Mater. Chem. A 2015, 3,894.
doi: 10.1039/C4TA05902F |
| [1] | Jiawei He, Liu Jiao, Xueyi Cheng, Guanghai Chen, Qiang Wu, Xizhang Wang, Lijun Yang, Zheng Hu. Structural Regulation of Metal Organic Framework-derived Hollow Carbon Nanocages and Their Lithium-Sulfur Battery Performance [J]. Acta Chimica Sinica, 2022, 80(7): 896-902. |
| [2] | Shouxiao Chen, Junke Liu, Weichen Zheng, Guozhen Wei, Yao Zhou, Juntao Li. Electron/ion Conductor Double-coated LiNi0.8Co0.1Mn0.1O2 Li-ion Battery Cathode Material and Its Electrochemical Performance [J]. Acta Chimica Sinica, 2022, 80(4): 485-493. |
| [3] | Qing Huang, Rui Ding, Lai Chen, Yun Lu, Qi Shi, Qiyu Zhang, Qijun Nie, Yuefeng Su, Feng Wu. Dual-Decoration and Mechanism Analysis of Ni-rich LiNi0.83Co0.11Mn0.06O2 Cathodes by Na2PO3F [J]. Acta Chimica Sinica, 2022, 80(2): 150-158. |
| [4] | Xiaolan Xue, Yang Zhang, Meiyu Shi, Tianlin Li, Tianlong Huang, Jiqiu Qi, Fuxiang Wei, Yanwei Sui, Zhong Jin. Recent Progress on Organic Electrode Materials for Nonaqueous Magnesium Secondary Batteries [J]. Acta Chimica Sinica, 2022, 80(12): 1618-1628. |
| [5] | Kai Qiu, Mingxia Yan, Shouwang Zhao, Shengli An, Wei Wang, Guixiao Jia. Theoretical Study on the Structural Stability and Oxygen Ion Oxidation of Al-doped Lithium-ion Battery Layered Cathode Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2 [J]. Acta Chimica Sinica, 2021, 79(9): 1146-1153. |
| [6] | Bixia Lin, Yingshan Huang, Shuai Chen, Zhenyu Xing. Research Progress of Key Materials for Sodium-selenium Batteries [J]. Acta Chimica Sinica, 2021, 79(5): 641-648. |
| [7] | Lu Zhang, Wenfeng Wang, Hongming Zhang, Shumin Han, Limin Wang. Research Progress and Challenge of Aqueous Zinc Ion Battery [J]. Acta Chimica Sinica, 2021, 79(2): 158-175. |
| [8] | Yanli Li, Dandan Yu, Sen Lin, Dongfei Sun, Ziqiang Lei. Preparation of α-MnO2 Nanorods/Porous Carbon Cathode for Aqueous Zinc-ion Batteries [J]. Acta Chimica Sinica, 2021, 79(2): 200-207. |
| [9] | Qimei Liang, Yujiao Guo, Junming Guo, Mingwu Xiang, Xiaofang Liu, Wei Bai, Ping Ning. Preparation and High Temperature Electrochemical Performance of LiNi0.08Mn1.92O4 Cathode Material of Submicron Truncated Octahedron [J]. Acta Chimica Sinica, 2021, 79(12): 1526-1533. |
| [10] | Jisheng Xie, Zhumei Xiao, Wenhua Zuo, Yong Yang. Research Progresses of Sodium Cobalt Oxide as Cathode in Sodium Ion Batteries [J]. Acta Chimica Sinica, 2021, 79(10): 1232-1243. |
| [11] | Tang Gong-ao, Mao Kun, Zhang Jing, Lyu Pin, Cheng Xueyi, Wu Qiang, Yang Lijun, Wang Xizhang, Hu Zheng. Hierarchical Nitrogen-doped Carbon Nanocages as High-rate Long-life Cathode Material for Rechargeable Magnesium Batteries [J]. Acta Chimica Sinica, 2020, 78(5): 444-450. |
| [12] | Liu Jiuding, Zhang Yudong, Liu Junxiang, Li Jinhan, Qiu Xiaoguang, Cheng Fangyi. In-situ Li3PO4 Coating of Li-Rich Mn-Based Cathode Materials for Lithium-ion Batteries [J]. Acta Chimica Sinica, 2020, 78(12): 1426-1433. |
| [13] | Ren Xuqiang, Li Donglin, Zhao Zhenzhen, Chen Guangqi, Zhao Kun, Kong Xiangze, Li Tongxin. Dual Effect of Aluminum Doping and Lithium Tungstate Coating on the Surface Improves the Cycling Stability of Lithium-rich Manganese-based Cathode Materials [J]. Acta Chimica Sinica, 2020, 78(11): 1268-1274. |
| [14] | Song Xuexi, Li Jicheng, Li Zhaohui, Li Xifei, Ding Yanhuai, Xiao Qizhen, Lei Gangtie. Effect of K-Doping on the Sodium-storage Performance of Sodium Vanadate Nanoplates [J]. Acta Chim. Sinica, 2019, 77(7): 625-633. |
| [15] | Li Zhao, Wang Zhong, Ban Liqin, Wang Jiantao, Lu Shigang. Recent Advances on Surface Modification of Li- and Mn-Rich Cathode Materials [J]. Acta Chimica Sinica, 2019, 77(11): 1115-1128. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||