Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (12): 1569-1575.DOI: 10.6023/A22090408 Previous Articles Next Articles
Article
投稿日期:2022-09-30
发布日期:2022-11-11
通讯作者:
罗群力
作者简介:基金资助:
Yucheng Yin, Lijing Leng, Xiaolong Lin, Yan Yu, Tian Cai, Qunli Luo( )
)
Received:2022-09-30
Published:2022-11-11
Contact:
Qunli Luo
About author:Supported by:Share
Yucheng Yin, Lijing Leng, Xiaolong Lin, Yan Yu, Tian Cai, Qunli Luo. One-Pot Synthesis of 1,4-Bridged Dihydroisoquinoline-3-ones from Isoquinolinium Salts and Cyclic 1,3-Diketones[J]. Acta Chimica Sinica, 2022, 80(12): 1569-1575.
| Entry | Additive (equiv.) | T/℃ | t/h | Yield/% |
|---|---|---|---|---|
| 1 | cyclopentane-1,3-dione (0.5) | 30 | 8 | 47 |
| 2 | cyclohexane-1,3-dione (0.5) | 30 | 8 | 75 |
| 3 | cyclohexane-1,3-dione (0.3) | 30 | 8 | 58 |
| 4 | cyclohexane-1,3-dione (0.5) | 20 | 10 | 64 |
| 5 | cyclohexane-1,3-dione (1) | 20 | 10 | 66 |
| 6b | TBHP (2) | 50 | 48 | 93 |
| Entry | Additive (equiv.) | T/℃ | t/h | Yield/% |
|---|---|---|---|---|
| 1 | cyclopentane-1,3-dione (0.5) | 30 | 8 | 47 |
| 2 | cyclohexane-1,3-dione (0.5) | 30 | 8 | 75 |
| 3 | cyclohexane-1,3-dione (0.3) | 30 | 8 | 58 |
| 4 | cyclohexane-1,3-dione (0.5) | 20 | 10 | 64 |
| 5 | cyclohexane-1,3-dione (1) | 20 | 10 | 66 |
| 6b | TBHP (2) | 50 | 48 | 93 |
| Entry | Acid (equiv.) | equiv. of 7a | T/℃ | tb/h | Yield/% | Entry | Acid (equiv.) | equiv. of 7a | T/℃ | tb/h | Yield/% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BF3•Et2O (0.2) | 2 | 50 | 4 | 66 | 9 | TfOH (0.3) | 2 | 50 | 5 | 77 |
| 2 | Cu(OTf)2 (0.2) | 2 | 50 | 6 | 64 | 10 | TfOH (0.4) | 2 | 50 | 5 | 77 |
| 3 | TFA (0.2) | 2 | 50 | 4 | 65 | 11 | TfOH (0.3) | 1.5 | 50 | 5 | 67 |
| 4 | TsOH (0.2) | 2 | 50 | 4 | 66 | 12 | TfOH (0.3) | 1.75 | 50 | 5 | 74 |
| 5 | MsOH (0.2) | 2 | 50 | 5 | 67 | 13 | TfOH (0.3) | 2.25 | 50 | 5 | 72 |
| 6 | TfOH (0.2) | 2 | 50 | 5 | 69 | 14 | TfOH (0.3) | 2 | 40 | 6 | 71 |
| 7c | TfOH (0.2) | 2 | 50 | 5 | 53 | 15 | TfOH (0.3) | 2 | 60 | 5 | 73 |
| 8d | TfOH (0.2) | 2 | 50 | 5 | 46 | 16 | TfOH (0.3) | 2 | 70 | 3 | 58 |
| Entry | Acid (equiv.) | equiv. of 7a | T/℃ | tb/h | Yield/% | Entry | Acid (equiv.) | equiv. of 7a | T/℃ | tb/h | Yield/% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BF3•Et2O (0.2) | 2 | 50 | 4 | 66 | 9 | TfOH (0.3) | 2 | 50 | 5 | 77 |
| 2 | Cu(OTf)2 (0.2) | 2 | 50 | 6 | 64 | 10 | TfOH (0.4) | 2 | 50 | 5 | 77 |
| 3 | TFA (0.2) | 2 | 50 | 4 | 65 | 11 | TfOH (0.3) | 1.5 | 50 | 5 | 67 |
| 4 | TsOH (0.2) | 2 | 50 | 4 | 66 | 12 | TfOH (0.3) | 1.75 | 50 | 5 | 74 |
| 5 | MsOH (0.2) | 2 | 50 | 5 | 67 | 13 | TfOH (0.3) | 2.25 | 50 | 5 | 72 |
| 6 | TfOH (0.2) | 2 | 50 | 5 | 69 | 14 | TfOH (0.3) | 2 | 40 | 6 | 71 |
| 7c | TfOH (0.2) | 2 | 50 | 5 | 53 | 15 | TfOH (0.3) | 2 | 60 | 5 | 73 |
| 8d | TfOH (0.2) | 2 | 50 | 5 | 46 | 16 | TfOH (0.3) | 2 | 70 | 3 | 58 |
| [1] |
(a) Li, K.; Ou, J.-J.; Gao, S. Angew. Chem., Int. Ed. 2016, 55, 14778.
|
|
(b) Wang, B.-X.; Xu, Z.-X.; Wu, J. Chin. J. Org. Chem. 2006, 26, 1587. (in Chinese)
|
|
|
王炳祥, 徐助雄, 吴婧, 有机化学 2006, 26, 1587.)
|
|
|
(c) Shi, L.; Ji, Y.; Huang, W.-X.; Zhou, Y.-G. Acta Chim. Sinica 2014, 72, 820. (in Chinese)
|
|
|
( 时磊, 姬悦, 黄文学, 周永贵, 化学学报 2014, 72, 820.)
doi: 10.6023/A14050391 |
|
| [2] |
Capacci, A. G.; Dechantsreiter, M.; Enyedy, I.; Jones, J. H.; Lin, E. Y.-S.; Lucas, B. S.; Ma, B. WO 2018140876, 2018
|
|
[Chem. Abstr. 2018, 169, 242866 ]
|
|
| [3] |
Tanifuji, R.; Minami, A.; Oguri, H.; Oikawa, H. Nat. Prod. Rep. 2020, 37, 1098.
doi: 10.1039/c9np00073a pmid: 32141467 |
| [4] |
Krishnan, S.; Bagdanoff, J. T.; Ebner, D. C.; Ramtohul, Y. K.; Tambar, U. K.; Stoltz, B. M. J. Am. Chem. Soc. 2008, 130, 13745.
doi: 10.1021/ja804738b pmid: 18798630 |
| [5] |
Le, V. H.; Inai, M.; Williams, R. M.; Kan, T. Nat. Prod. Rep. 2015, 32, 328.
doi: 10.1039/c4np00051j pmid: 25273374 |
| [6] |
(a) Ding, B.-D.; Jiang, Y.-C.; Zhang, Y.; Ye, R.; Sun, J.; Yan, C.-G. Chin. J. Org. Chem. 2020, 40, 1003. (in Chinese)
|
|
( 丁邦东, 姜业朝, 张瑜, 叶蓉, 孙晶, 颜朝国, 有机化学 2020, 40, 1003.)
doi: 10.6023/cjoc201910016 |
|
|
(b) Shen, G.-L.; Sun, J.; Yan, C.-G. Chin. J. Chem. 2016, 34, 412.
|
|
|
(c) Niu, F.; Cui, Z.; Chang, H.-T.; Jiang, Y.; Chen, F.-K.; Tu, P.-F. Chin. J. Chem. 2006, 24, 1788.
|
|
|
(d) Wang, L.-L.; Zhang, Z.-Y.; Han, H.-B.; Liu, X.-L.; Bu, Z.-W.; Wang, Q.-L. Chin. J. Org. Chem. 2021, 41, 12. (in Chinese)
|
|
|
( 王乐乐, 张子莹, 韩华彬, 刘雄利, 卜站伟, 王琪琳, 有机化学 2021, 41, 12.)
doi: 10.6023/cjoc202007045 |
|
|
(e) Das, S. Org. Biomol. Chem. 2022, 20, 1838.
|
|
|
(f) Zhang, Z.-Y.; Han, H.-B.; Wang, L.-L.; Bu, Z.-W.; Xie, Y.; Wang, Q.-L. Org. Biomol. Chem. 2021, 19, 3960.
|
|
|
(g) Sharma, U. K.; Ranjan, P.; Van der Eycken, E. V.; You, S.-L. Chem. Soc. Rev. 2020, 49, 8721.
|
|
|
(h) Xu, J.-H.; Zheng, S.-C.; Zhang, J.-W.; Liu, X.-Y.; Tan, B. Angew. Chem., Int. Ed. 2016, 55, 11834.
|
|
|
(i) Bai, X.-G.; Miao, H.-J.; Zhao, Y.; Wang, Q.-L.; Bu, Z.-W. Org. Lett. 2020, 22, 5068.
|
|
|
(j) Miao, H.-J.; Wang, L.-L.; Han, H.-B.; Zhao, Y.-D.; Wang, Q.-L.; Bu, Z.-W. Chem. Sci. 2020, 11, 1418.
|
|
|
(k) Miao, H.-J.; Bai, X.-G.; Wang, L.-L.; Yu, J.-H.; Wang, Q.-L.; Bu, Z.-W. Org. Chem. Front. 2021, 8, 204.
|
|
| [7] |
Lin, X.-L.; Yu, Y.; Zhang, L.; Leng, L.-J.; Xiao, D.-R.; Cai, T.; Luo, Q.-L. Org. Chem. Front. 2022, 9, 4676.
|
| [8] |
For details, see the Supporting Information.
|
| [9] |
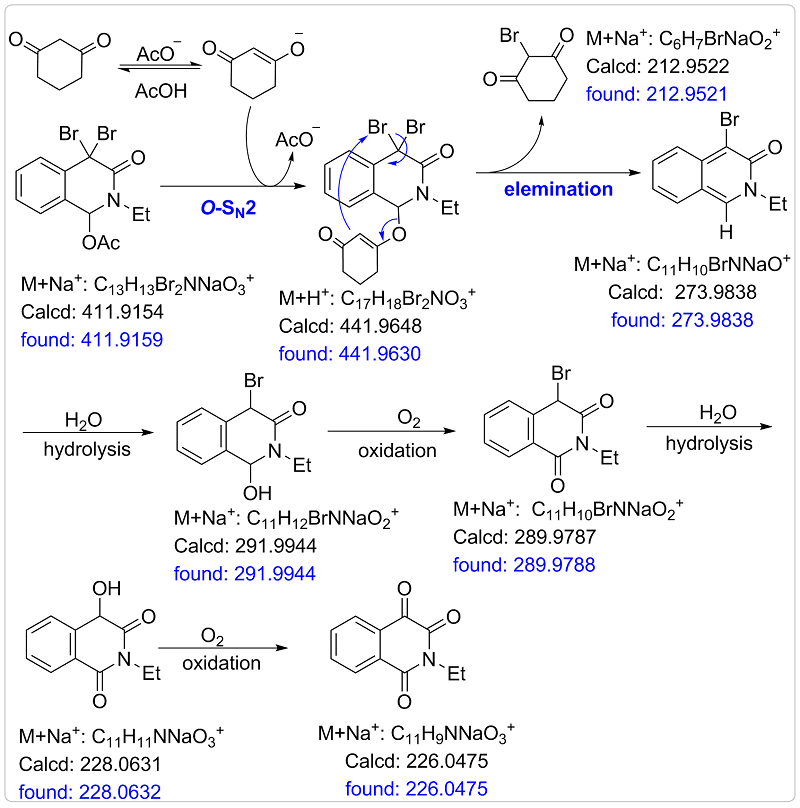
Increasing the reaction temperature did not improve the yield of isoquinoline-1,3,4-triones (also see the section of 2.2.1). Therefore, O-SN1 could be ruled out.
|
| [10] |
Bai, L.-G.; Zhou, Y.; Zhuang, X.; Zhang, L.; Xue, J.; Lin, X.-L.; Cai, T.; Luo, Q.-L. Green Chem. 2020, 22, 197.
|
| [11] |
Wang, S.-K.; Chen, M.-T.; Zhao, D.-Y.; You, X.; Luo, Q.-L. Adv. Synth. Catal. 2016, 358, 4093.
|
| [1] | Ping Li, Qiyu Yang, Jing Zeng, Ran Zhang, Qiuyan Chen, Fei Yan. Effect of Fluorine Doping on the Performance of Reversible Solid Oxide Cells and Related Kinetic Studies [J]. Acta Chimica Sinica, 2024, 82(1): 36-45. |
| [2] | Xinpu Fu, Xiuling Wang, Weiwei Wang, Rui Si, Chunjiang Jia. Fabrication and Mechanism Study of Clustered Au/CeO2 Catalyst for the CO Oxidation Reaction★ [J]. Acta Chimica Sinica, 2023, 81(8): 874-883. |
| [3] | Jianchuan Liu, Cuiyan Li, Yaozu Liu, Yujie Wang, Qianrong Fang. Highly-Stable Two-Dimensional Bicarbazole-based sp2-Carbon-conjugated Covalent Organic Framework for Efficient Electrocatalytic Oxygen Reduction★ [J]. Acta Chimica Sinica, 2023, 81(8): 884-890. |
| [4] | Xu Yuanli, Pan Hui, Yang Yi, Zuo Zhiwei. Selectively Aerobic Oxidation of Benzylic C—H Bonds Enabled by Dual Anthracene and Cerium Catalysis under Continuous-Flow Conditions★ [J]. Acta Chimica Sinica, 2023, 81(5): 435-440. |
| [5] | Huiying Zhang, Shuyan Yu, Congju Li. Electrocatalytic Degradation of Wastewater by Polymer-based Carbon Nanomembranes and Mechanism [J]. Acta Chimica Sinica, 2023, 81(4): 420-430. |
| [6] | Wentao Wang, Xinting Lai, Shiquan Yan, Lei Zhu, Yuyuan Yao, Liming Ding. Synergistic Treatment of Dye Wastewater by the Adsorption-Degradation of a Bifunctional Aerogel [J]. Acta Chimica Sinica, 2023, 81(3): 222-230. |
| [7] | Luyao Yu, Zhen Ren, Yusen Yang, Min Wei. Directed Preparation of Biomass-based Polyester Monomers by Catalytic Conversion [J]. Acta Chimica Sinica, 2023, 81(2): 175-190. |
| [8] | Yao Li, Bingnian Chen, Dan Luo, Shan Lei, Li Wang. Study on the Antioxidant Properties of Polyoxometalates α-Glucosidase Inhibitors [J]. Acta Chimica Sinica, 2023, 81(10): 1318-1326. |
| [9] | Yahui Jia, Chunsheng Li, Zhongzhen Xu, Wei Liu, Daowei Gao, Guozhu Chen. The SMSI of Pt-TiO2 During the Crystalline Phase Transformation and Its Effect on CO Oxidation Performance [J]. Acta Chimica Sinica, 2022, 80(9): 1289-1298. |
| [10] | Yige Wang, Hangyue Li, Zewei Lyu, Minfang Han, Kaihua Sun. Study of Operating Conditions for High Efficiency and Anode Safety of Industrial-Size Solid Oxide Fuel Cell [J]. Acta Chimica Sinica, 2022, 80(8): 1091-1099. |
| [11] | Yanfu Liu, Shilin Li, Yanan Jing, Linge Xiao, Huiqiong Zhou. Research Progress in Degradation Mechanism of Organic Solar Cells [J]. Acta Chimica Sinica, 2022, 80(7): 993-1009. |
| [12] | Jinge Wang, Wei Zhou, Jiayi Li, Yani Ding, Jihui Gao. Recent Advances and Performance Enhancement Mechanisms of Pulsed Electrocatalysis [J]. Acta Chimica Sinica, 2022, 80(11): 1555-1568. |
| [13] | Pengfei Zhu, Chensi Lou, Yuhan Shi, Chuanyi Wang. Study on Preparation of Ag/AgCl/ZIF-8 Composite and Photocatalytic NO Oxidation Performance [J]. Acta Chimica Sinica, 2022, 80(10): 1385-1393. |
| [14] | Kai Qiu, Mingxia Yan, Shouwang Zhao, Shengli An, Wei Wang, Guixiao Jia. Theoretical Study on the Structural Stability and Oxygen Ion Oxidation of Al-doped Lithium-ion Battery Layered Cathode Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2 [J]. Acta Chimica Sinica, 2021, 79(9): 1146-1153. |
| [15] | Yang Zhongjie, Zhang Xiaofei, Shi Yanan, Long Chang, Zhang Binhao, Yan Shuhao, Chang Lin, Tang Zhiyong. Synthesis of Two-dimensional Hydrophobic Copper-based Nanosheets and Their Application in Catalytic Oxidation of Sulfides [J]. Acta Chimica Sinica, 2020, 78(9): 980-988. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||