Acta Chimica Sinica ›› 2023, Vol. 81 ›› Issue (1): 64-83.DOI: 10.6023/A22100422 Previous Articles Next Articles
Review
王晓晨a, 季泽尧a, 刘健a, 王炳福a,b, 金辉a,*( ), 张立新a,*(
), 张立新a,*( )
)
投稿日期:2022-10-10
发布日期:2022-12-05
作者简介: |
王晓晨, 1994年出生于山东菏泽, 2018年在山东第一医科大学获得学士学位, 2020年至今在张立新教授和金辉博士的指导下攻读硕士学位. 研究兴趣是发展以硫酯为底物的合成方法学合成具有潜在生物活性的分子. |
 |
季泽尧, 1998年出生于河南南阳, 2021年毕业于吉林化工学院制药工程专业获得学士学位. 2021年至今在张立新教授和金辉博士的指导下攻读硕士学位. 研究兴趣是发展新的合成方法学合成具有潜在生物活性的分子. |
 |
刘健, 1996年出生于辽宁鞍山, 2019年在辽宁科技大学获得学士学位. 2021年至今在张立新教授和金辉博士的指导下攻读硕士学位. 研究兴趣是发展新的合成方法学合成具有潜在生物活性的分子. |
 |
王炳福, 1992年出生于黑龙江哈尔滨, 2015年和2018年在辽宁科技大学先后获得学士和硕士学位, 2019年至今, 在张立新教授和金辉博士的指导下攻读博士学位. 研究方向为不对称有机小分子催化. |
 |
金辉讲师、硕士生导师. 1989年出生于辽宁丹东, 2011年在大连医科大学获得学士学位(药学). 2014年在沈阳药科大学获得硕士学位(药物化学). 2014年至2018年在韩国成均馆大学化学系学习并获得有机化学博士学位(导师:Do Hyun Ryu教授). 2018年加入沈阳化工大学功能分子研究所从事科研教学工作. 研究兴趣主要是发展新的合成方法学合成具有潜在生物活性的分子. |
 |
张立新教授、博士生导师. 1966年出生于辽宁锦州, 1987年在兰州大学获得学士学位. 1993年在沈阳化工研究院获得硕士学位. 2000年至2004年在英国利兹大学化学系学习并获得有机化学博士学位(导师:Ronald Grigg教授). 曾就职于沈阳化工研究院(1987~2000, 高级工程师)、2016年加入沈阳化工大学, 组建功能分子研究所. 研究兴趣主要是新农药、医药创制和有机合成方法学. |
基金资助:
Xiaochen Wanga, Zeyao Jia, Jian Liua, Bingfu Wanga,b, Hui Jina( ), Lixin Zhanga(
), Lixin Zhanga( )
)
Received:2022-10-10
Published:2022-12-05
Contact:
*E-mail: Supported by:Share
Xiaochen Wang, Zeyao Ji, Jian Liu, Bingfu Wang, Hui Jin, Lixin Zhang. Advances in Organocatalytic Asymmetric Reactions Involving Thioesters[J]. Acta Chimica Sinica, 2023, 81(1): 64-83.
| 序号 | 标题 |
|---|---|
| 1 | 引言 |
| 2 | 可烯醇化硫酯参与的不对称反应 |
| 2.1 | 多氟烷基取代硫酯参与的反应 |
| 2.2 | α-羰基取代硫酯参与的反应 |
| 2.2.1 | 丙二酸半硫酯参与的反应 |
| 2.2.2 | 丙二酸单硫酯参与的反应 |
| 2.2.3 | 丙二酸二硫酯和β-酮硫酯参与的反应 |
| 2.2.4 | α-氮取代乙酸硫酯参与的反应 |
| 3 | α,β-不饱和硫酯参与的不对称反应 |
| 3.1 | 非共价催化α,β-不饱和硫酯的反应 |
| 3.2 | 共价催化α,β-不饱和硫酯的反应 |
| 4 | 总结与展望 |
| 序号 | 标题 |
|---|---|
| 1 | 引言 |
| 2 | 可烯醇化硫酯参与的不对称反应 |
| 2.1 | 多氟烷基取代硫酯参与的反应 |
| 2.2 | α-羰基取代硫酯参与的反应 |
| 2.2.1 | 丙二酸半硫酯参与的反应 |
| 2.2.2 | 丙二酸单硫酯参与的反应 |
| 2.2.3 | 丙二酸二硫酯和β-酮硫酯参与的反应 |
| 2.2.4 | α-氮取代乙酸硫酯参与的反应 |
| 3 | α,β-不饱和硫酯参与的不对称反应 |
| 3.1 | 非共价催化α,β-不饱和硫酯的反应 |
| 3.2 | 共价催化α,β-不饱和硫酯的反应 |
| 4 | 总结与展望 |
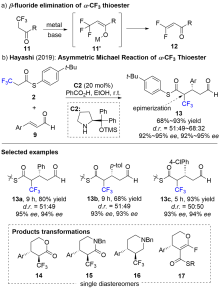

| [1] |
(a) Ding, K. L.; Fan, Q. H. Asymmetric Catalysis: New Concepts and Methods, Chemical Industry Press, Beijing, 2009. (in Chinese)
|
|
( 丁奎岭, 范青华, 不对称催化新概念与新方法, 化学工业出版社, 北京, 2009.)
|
|
|
(b) Ding, K. L. Acta Chim. Sinica 2014, 72, 755. (in Chinese)
doi: 10.6023/A1407E001 |
|
|
( 丁奎岭, 化学学报 2014, 72, 755.)
doi: 10.6023/A1407E001 |
|
|
(c) Dalko, P. I. Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications, Wiley-VCH Verlag GmbH, Weinheim, 2013.
|
|
| [2] |
Saha, D.; Kharbanda, A.; Yan, W.; Lakkaniga, N. R.; Frett, B.; Li, H.-y. J. Med. Chem. 2020, 63, 441.
doi: 10.1021/acs.jmedchem.9b00640 |
| [3] |
(a) Mulholland, A. J.; Lyne, P. D.; Karplus, M. J. Am. Chem. Soc. 2000, 122, 534.
doi: 10.1021/ja992874v pmid: 8011640 |
|
(b) Usher, K. C.; Remington, S. J.; Martin, D. P.; Drueckhammer, D. G. Biochemistry 1994, 33, 7753.
pmid: 8011640 |
|
|
(c) Wiegand, G.; Remington, S. J. Ann. Rev. Biophys. Chem. 1986, 15, 97.
pmid: 8011640 |
|
| [4] |
(a) Hill, A. M. Nat. Prod. Rep. 2006, 23, 256.
doi: 10.1039/B301028G pmid: 11548049 |
|
(b) Staunton, J.; Weissman, K. J. Nat. Prod. Rep. 2001, 18, 380.
doi: 10.1039/a909079g pmid: 11548049 |
|
| [5] |
(a) Masamune, S.; Kamata, S.; Schilling, W. J. Am. Chem. Soc. 1975, 97, 3515.
pmid: 893906 |
|
(b) Masamune, S.; Hayase, Y.; Schilling, W.; Chan, W. K.; Bates, G. S. J. Am. Chem. Soc. 1977, 99, 6756.
pmid: 893906 |
|
| [6] |
Yang, X.; Ma, Y.; Di, H.; Wang, X.; Jin, H.; Ryu, D. H.; Zhang, L. Adv. Synth. Catal. 2021, 363, 3201.
doi: 10.1002/adsc.202100369 |
| [7] |
(a) Tokuyama, H.; Yokoshima, S.; Yamashita, T.; Fukuyama, T. Tetrahedron Lett. 1998, 39, 3189.
|
|
(b) Miyazaki, T.; Han-ya, Y.; Tokuyama, H.; Fukuyama, T. Synlett 2004, 477.
|
|
|
(c) Fukuyama, T.; Tokuyama, H. Aldrichimica Acta 2004, 37, 87.
|
|
|
(d) Mori, Y.; Seki, M. Adv. Synth. Catal. 2007, 349, 2027.
doi: 10.1002/adsc.200600610 |
|
|
(e) Cherney, A. H.; Reisman, S. E. Tetrahedron 2014, 70, 3259.
doi: 10.1016/j.tet.2013.11.104 |
|
| [8] |
Um, P.-J.; Drueckhammer, D. G. J. Am. Chem. Soc. 1998, 120, 5607.
|
| [9] |
(a) Hirschbeck, V.; Gehrtz, P. H.; Fleischer, I. Chem. Eur. J. 2018, 24, 7092.
doi: 10.1002/chem.201705025 |
|
(b) Ye, Q.; Cao, W.-G.; Gao, J.-S. Chin. J. Org. Chem. 2002, 21, 697. (in Chinese)
|
|
|
( 叶青, 曹卫国, 高金森, 有机化学, 2002, 21, 697.)
|
|
| [10] |
(a) Magdziak, D.; Lalic, G.; Lee, M. H.; Fortner, K. C.; Aloise, A. D.; Shair, M. D. J. Am. Chem. Soc. 2005, 127, 7284.
pmid: 15898756 |
|
(b) Orlandi, S.; Benaglia, M.; Cozzi, F. Tetrahedron Lett. 2004, 45, 1747.
pmid: 15898756 |
|
|
(c) Sawa, M.; Miyazaki, S.; Yonesaki, R.; Morimoto, H.; Ohshima, T. Org. Lett. 2018, 20, 5393.
doi: 10.1021/acs.orglett.8b02306 pmid: 15898756 |
|
|
(d) Furutachi, M.; Mouri, S.; Matsunaga, S.; Shibasaki, M. Chem. Asian J. 2010, 5, 2351.
doi: 10.1002/asia.201000540 pmid: 15898756 |
|
| [11] |
Walker, M. C.; Thuronyi, B. W.; Charkoudian, L. K.; Lowry, B.; Khosla, C.; Chang, M. C. Y. Science 2013, 341, 1089.
doi: 10.1126/science.1242345 pmid: 24009388 |
| [12] |
(a) Xiang, S.-H.; Tan, B. Nat. Commun. 2020, 11, 3786.
doi: 10.1038/s41467-020-17580-z |
|
(b) Zhang, M.-M.; Luo, Y.-Y.; Lu, L.-Q.; Xiao, W.-J. Acta Chim. Sinica 2018, 76, 838. (in Chinese)
doi: 10.6023/A18060237 |
|
|
( 张毛毛, 骆元元, 陆良秋, 肖文精, 化学学报 2018, 76, 838.)
doi: 10.6023/A18060237 |
|
|
(c) Di, H.; Liu, Y.; Ma, Y.; Yang, X.; Jin, H.; Zhang, L. Chin. J. Org. Chem. 2021, 41, 2228. (in Chinese)
doi: 10.6023/cjoc202010039 |
|
|
( 底慧明, 刘云婷, 马艳榕, 杨鑫悦, 金辉, 张立新, 有机化学, 2021, 41, 2228.)
doi: 10.6023/cjoc202010039 |
|
|
(d) Ren, H.; Ma, M.; Huang, Y. Chin. J. Org. Chem. 2022, 42, 3129. (in Chinese)
doi: 10.6023/cjoc202208024 |
|
|
任红霞, 马萌萌, 黄有, 有机化学, 2022, 42, 3129.).
doi: 10.6023/cjoc202208024 |
|
|
(e) Chen, X.; Wang, H.; Jin, Z.; Chi, Y. R. Chin. J. Chem. 2020, 38, 1167.
doi: 10.1002/cjoc.202000107 |
|
| [13] |
Bordwell, F. G.; Fried, H. E. J. Org. Chem. 1991, 56, 4218.
doi: 10.1021/jo00013a027 |
| [14] |
Um, P.-J.; Drueckhammer, D. G. J. Am. Chem. Soc. 1998, 120, 5605.
doi: 10.1021/ja980445b |
| [15] |
Alonso, D. A.; Kitagaki, S.; Utsumi, N.; Barbas III, C. F. Angew. Chem., Int. Ed. 2008, 47, 4588.
doi: 10.1002/anie.200801088 pmid: 18464230 |
| [16] |
Hayashi, Y.; Yamada, T.; Sato, M.; Watanabe, S.; Kwon, E.; Iwasaki, K.; Umemiya, S. Org. Lett. 2019, 21, 5183.
doi: 10.1021/acs.orglett.9b01774 pmid: 31247799 |
| [17] |
Kohler, M. C.; Yost, J. M.; Garnsey, M. R.; Coltart, D. M. Org. Lett. 2010, 12, 3376.
doi: 10.1021/ol101152b pmid: 20608684 |
| [18] |
Clayden, J.; Greeves, N.; Warren, S. Organic Chemistry, 2nd ed., Oxford University Press, New York, 2012, pp. 628-630.
|
| [19] |
Kobuke, Y.; Yoshida, J.-i. Tetrahedron Lett. 1978, 19, 367.
doi: 10.1016/S0040-4039(01)85127-3 |
| [20] |
(a) Magdziak, D.; Lalic, G.; Lee, H. M.; Fortner, K. C.; Aloise, A. D.; Shair, M. D. J. Am. Chem. Soc. 2005, 127, 7284.
pmid: 17263375 |
|
(b) Fortner, K. C.; Shair, M. D. J. Am. Chem. Soc. 2007, 129, 1032.
pmid: 17263375 |
|
|
(c) Lalic, G.; Aloise, A. D.; Shair, M. D. J. Am. Chem. Soc. 2003, 125, 2852.
doi: 10.1021/ja029452x pmid: 17263375 |
|
|
(d) Orlandi, S.; Benaglia, M.; Cozzi, F. Tetrahedron Lett. 2004, 45, 1747.
pmid: 17263375 |
|
| [21] |
Bae, H. Y.; Sim, J. H.; Lee, J.-W.; List, B.; Song, C. E. Angew. Chem., Int. Ed. 2013, 52, 12143.
doi: 10.1002/anie.201306297 |
| [22] |
Wang, Y.; Huang, G.; Hu, S.; Jin, K.; Wu, Y.; Chen, F. Tetrahedron Lett. 2017, 73, 5055.
doi: 10.1016/j.tet.2017.05.066 |
| [23] |
(a) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
doi: 10.1126/science.1131943 pmid: 18197347 |
|
(b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 32.
pmid: 18197347 |
|
|
(c) O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308.
doi: 10.1039/b711844a pmid: 18197347 |
|
| [24] |
Saadi, J.; Akakura, M.; Yamamoto, H. J. Am. Chem. Soc. 2011, 133, 14248.
doi: 10.1021/ja2066169 |
| [25] |
Saadi, J.; Wennemers, H. Nat. Chem. 2016, 8, 276.
doi: 10.1038/nchem.2437 |
| [26] |
Zetschok, D.; Heieck, L.; Wennemers, H. Org. Lett. 2021, 23, 1753.
doi: 10.1021/acs.orglett.1c00172 pmid: 33591192 |
| [27] |
(a) Bao, Y.; Kumagai, N.; Shibasaki, M. Chem. Sci. 2015, 6, 6124.
doi: 10.1039/C5SC02218E pmid: 19722664 |
|
(b) Misaki, T.; Takimoto, G.; Sugimura, T. J. Am. Chem. Soc. 2010, 132, 6286.
doi: 10.1021/ja101216x pmid: 19722664 |
|
|
(c) Yamaguchi, A.; Matsunaga, S.; Shibasaki, M. J. Am. Chem. Soc. 2009, 131, 10842.
doi: 10.1021/ja904575e pmid: 19722664 |
|
|
(d) Saito, S.; Kobayashi, S. J. Am. Chem. Soc. 2006, 128, 8704.
doi: 10.1021/ja061221t pmid: 19722664 |
|
|
(e) Suto, Y.; Tsuji, R.; Kanai, M.; Shibasaki, M. Org. Lett. 2005, 7, 3757.
doi: 10.1021/ol051423e pmid: 19722664 |
|
| [28] |
Park, J. H.; Sim, J. H.; Song, C. E. Org. Lett. 2019, 21, 4567.
doi: 10.1021/acs.orglett.9b01469 |
| [29] |
Ricci, A.; Pettersen, D.; Bernardi, L.; Fini, F.; Fochi, F.; Herrera, R. P.; Sgarzania, V. Adv. Synth. Catal. 2007, 349, 1037.
doi: 10.1002/adsc.200600536 |
| [30] |
Pan, Y. H.; Kee, C. W.; Jiang, Z. Y.; Ma, T.; Zhao, Y. J.; Yang, Y. Y.; Xue, H. S.; Tan, C. H. Chem. Eur. J. 2011, 17, 8363.
doi: 10.1002/chem.201100687 |
| [31] |
Hara, N.; Nakamura, S.; Sano, M. Chem. Eur. J. 2012, 18, 9276.
doi: 10.1002/chem.201200367 |
| [32] |
(a) Takashina, N.; Price, C. C. J. Am. Chem. Soc. 1962, 84, 489.
doi: 10.1021/ja00862a034 |
|
(b) Rauhut, M. M.; Currier, H. A.; Semsel, A. M.; Wystrach, V. P. J. Org. Chem. 1961, 26, 5138.
doi: 10.1021/jo01070a087 |
|
|
(c) Morita, K.-i.; Suzuki, Z.; Hirose, H. Bull. Chem. Soc. Jpn. 1968, 41, 2815.
doi: 10.1246/bcsj.41.2815 |
|
| [33] |
(a) Lu, X.; Zhang, C.; Xu, Z. Acc. Chem. Res. 2001, 34, 535.
doi: 10.1021/ar000253x |
|
(b) Methot, J. L.; Roush, W. R. Adv. Synth. Catal. 2004, 346, 1035.
doi: 10.1002/adsc.200404087 |
|
|
(c) Lu, X.; Du, Y.; Lu, C. Pure Appl. Chem. 2005, 77, 1985.
doi: 10.1351/pac200577121985 |
|
|
(d) Ye, L.-W.; Zhou, J.; Tang, Y. Chem. Soc. Rev. 2008, 37, 1140.
doi: 10.1039/b717758e |
|
|
(e) Cowen, B. J.; Miller, S. J. Chem. Soc. Rev. 2009, 38, 3102.
doi: 10.1039/b816700c |
|
|
(f) Wei, Y.; Shi, M. Acc. Chem. Res. 2010, 43, 1005.
doi: 10.1021/ar900271g |
|
|
(g) Marinetti, A.; Voituriez, A. Synlett 2010, 2010, 174.
doi: 10.1055/s-0029-1219157 |
|
|
(h) Lu, Y.; Wang, S.-X.; Han, X.; Zhong, F.; Wang, Y. Synlett 2011, 2011, 2766.
doi: 10.1055/s-0031-1289538 |
|
|
(i) Zhao, Q.-Y.; Lian, Z.; Wei, Y.; Shi, M. Chem. Commun. 2012, 48, 1724.
doi: 10.1039/C1CC15793K |
|
|
(j) Fan, Y. C.; Kwon, O. Chem. Commun. 2013, 49, 11588.
doi: 10.1039/c3cc47368f |
|
|
(k) Wang, Z.; Xu, X.; Kwon, O. Chem. Soc. Rev. 2014, 43, 2927.
doi: 10.1039/C4CS00054D |
|
|
(l) Xiao, Y.; Sun, Z.; Guo, H.; Kwon, O. J. Org. Chem. 2014, 10, 2089.
|
|
|
(m) Wang, T.; Han, X.; Zhong, F.; Yao, W.; Lu, Y. Acc. Chem. Res. 2016, 49, 1369.
doi: 10.1021/acs.accounts.6b00163 |
|
|
(n) Li, W.; Zhang, J. Chem. Soc. Rev. 2016, 45, 1657.
doi: 10.1039/C5CS00469A |
|
|
(o) Ni, H.; Chan, W.-L.; Lu, Y. Chem. Rev. 2018, 118, 9344.
doi: 10.1021/acs.chemrev.8b00261 |
|
|
(p) Guo, H.; Fan, Y. C.; Sun, Z.; Wu, Y.; Kwon, O. Chem. Rev. 2018, 118, 10049.
doi: 10.1021/acs.chemrev.8b00081 |
|
| [34] |
(a) White, D. A.; Baizer, M. M. Tetrahedron Lett. 1973, 14, 3597.
doi: 10.1016/S0040-4039(01)86980-X pmid: 23339132 |
|
(b) Trost, B. M.; Li, C.-J. J. Am. Chem. Soc. 1994, 116, 10819.
doi: 10.1021/ja00102a071 pmid: 23339132 |
|
|
(c) Trost, B. M.; Li, C.-J. J. Am. Chem. Soc. 1994, 116, 3167.
doi: 10.1021/ja00086a074 pmid: 23339132 |
|
|
(d) Trost, B. M.; Dake, G. R. J. Org. Chem. 1997, 62, 5670.
doi: 10.1021/jo970848e pmid: 23339132 |
|
|
(e) Zhang, C.; Lu, X. Synlett 1995, 1995, 645.
doi: 10.1055/s-1995-5042 pmid: 23339132 |
|
|
(f) Chung, Y. K.; Fu, G. C. Angew. Chem., Int. Ed. 2009, 48, 2225.
doi: 10.1002/anie.200805377 pmid: 23339132 |
|
|
(g) Smith, S. W.; Fu, G. C. J. Am. Chem. Soc. 2009, 131, 14231.
doi: 10.1021/ja9061823 pmid: 23339132 |
|
|
(h) Sun, J.; Fu, G. C. J. Am. Chem. Soc. 2010, 132, 4568.
doi: 10.1021/ja101251d pmid: 23339132 |
|
|
(i) Lundgren, R. J.; Wilsily, A.; Marion, N.; Ma, C.; Chung, Y. K.; Fu, G. C. Angew. Chem., Int. Ed. 2013, 52, 2525.
doi: 10.1002/anie.201208957 pmid: 23339132 |
|
|
(j) Chen, Z.; Zhu, G.; Jiang, Q.; Xiao, D.; Cao, P.; Zhang, X. J. Org. Chem. 1998, 63, 5631.
doi: 10.1021/jo9721756 pmid: 23339132 |
|
| [35] |
(a) Zhong, F.; Dou, X.; Han, X.; Yao, W.; Zhu, Q.; Meng, Y.; Lu, Y. Angew. Chem., Int. Ed. 2013, 52, 943.
doi: 10.1002/anie.201208285 |
|
(b) Wang, T.; Yao, W.; Zhong, F.; Pang, G. H.; Lu, Y. Angew. Chem., Int. Ed. 2014, 126, 3008.
doi: 10.1002/ange.201307757 |
|
|
(c) Wang, T.; Yu, Z.; Hoon, D. L.; Huang, K.-W.; Lan, Y.; Lu, Y. Chem. Sci. 2015, 6, 4912.
doi: 10.1039/C5SC01614B |
|
|
(d) Wang, T.; Hoon, D. L.; Lu, Y. Chem. Commun. 2015, 51, 10186.
doi: 10.1039/C5CC03289J |
|
|
(e) Huang, G.; Ren, X.; Jiang, C.; Wu, J.-H.; Gao, G.; Wang, T. Org. Chem. Front. 2019, 6, 2872.
doi: 10.1039/C9QO00478E |
|
| [36] |
Wang, X.; Fang, F.; Zhao, C.; Tian, S.-K. Tetrahedron Lett. 2008, 49, 6442.
doi: 10.1016/j.tetlet.2008.08.092 |
| [37] |
(a) Wang, H.-Y.; Zhang, K.; Zheng, C.-W.; Chai, Z.; Cao, D.-D.; Zhang, J.-X.; Zhao, G. Angew. Chem., Int. Ed. 2015, 54, 1775.
doi: 10.1002/anie.201409342 |
|
(b) Lou, Y.-P.; Zheng, C.-W.; Pan, R.-M.; Jin, Q.-W.; Zhao, G.; Li, Z. Org. Lett. 2015, 17, 688.
doi: 10.1021/ol503712m |
|
|
(c) Xu, L.; Wang, H.; Zheng, C.; Zhao, G. Adv. Synth. Catal. 2017, 359, 2942.
doi: 10.1002/adsc.201700321 |
|
|
(d) Pan, R.; Zhang, J.; Zheng, C.; Wang, H.; Cao, D.; Cao, W.; Zhao, G. Tetrahedron 2017, 73, 2349.
doi: 10.1016/j.tet.2017.03.005 |
|
|
(e) Ji, X.; Cao, W.-G.; Zhao, G. Tetrahedron 2017, 73, 5983.
doi: 10.1016/j.tet.2017.08.031 |
|
|
(f) Jin, Q.; Zheng, C.; Zhao, G.; Zou, G. Tetrahedron 2018, 74, 4134.
doi: 10.1016/j.tet.2018.06.030 |
|
|
(g) Wang, H.-Y.; Zheng, C.-W.; Chai, Z.; Zhang, J.-X.; Zhao, G. Nat. Commun. 2016, 7, 12720.
doi: 10.1038/ncomms12720 |
|
| [38] |
Zhang, H.; Jiang, C.; Tan, J.-P.; Hu, H.-L.; Chen, Y.; Ren, X.; Zhang, H.-S.; Wang, T. ACS Catal. 2020, 10, 5698.
doi: 10.1021/acscatal.0c01079 |
| [39] |
(a) Fleming, F. F. Nat. Prod. Rep. 1999, 16, 597.
doi: 10.1039/a804370a |
|
(b) Miller, J. S.; Manson, J. L. Acc. Chem. Res. 2001, 34, 563.
doi: 10.1021/ar0000354 |
|
|
(c) Lindquist, B. A.; Furse, K. E.; Corcelli, S. A. Phys. Chem. 2009, 11, 8119.
|
|
| [40] |
(a) Laine, D.; Palovich, M.; McCleland, B.; Petitjean, E.; Delhom, I.; Xie, H.; Deng, J.; Lin, G.; Davis, R.; Jolit, A.; Nevins, N.; Zhao, B.; Villa, J.; Schneck, J.; McDevitt, P.; Midgett, R.; Kmett, C.; Umbrecht, S.; Peck, B.; Davis, A.-B.; Bettoun, D. ACS Med. Chem. Lett. 2011, 2, 142.
doi: 10.1021/ml100212k pmid: 30423248 |
|
(b) Raja, V.-P.-A.; Perumal, S.; Yogeeswari, P.; Sriram, D. Bioorg. Med. Chem. Lett. 2011, 21, 3881.
doi: 10.1016/j.bmcl.2011.05.032 pmid: 30423248 |
|
|
(c) Casimiro-Garcia, A.; Trujillo, J.-I.; Vajdos, F.; Juba, B.; Banker, M.-E.; Aulabaugh, A.; Balbo, P.; Bauman, J.; Chrencik, J.; Coe, J.-W.; Czerwinski, R.; Dowty, M.; Knafels, J.-D.; Kwon, S.; Leung, L.; Liang, S.; Robinson, R.-P.; Telliez, J.-B.; Unwalla, R.; Yang, X.; Thorarensen, A. J. Med. Chem. 2018, 61, 10665.
doi: 10.1021/acs.jmedchem.8b01308 pmid: 30423248 |
|
| [41] |
Oyamada, Y.; Inaba, K.; Sasamori, T.; Nakamura, S. Chem. Commun. 2022, 58, 2172.
doi: 10.1039/D1CC07191B |
| [42] |
Lubkoll, J.; Wennemers, H. Angew. Chem., Int. Ed. 2007, 46, 6841.
doi: 10.1002/anie.200702187 |
| [43] |
Bae, H. Y.; Some, S.; Lee, J. H.; Kim, J.-Y.; Song, M. J.; Lee, S.; Zhang, Y. J.; Song, C. E. Adv. Synth. Catal. 2011, 353, 3196.
doi: 10.1002/adsc.201100458 |
| [44] |
(a) Chiu, P.; Leung, L. T.; Ko, B. C. B. Nat. Prod. Rep. 2010, 27, 1066.
doi: 10.1039/b906520m pmid: 18389141 |
|
(b) Florence, G. J.; Gardnerb, N. M.; Paterson, I. Nat. Prod. Rep. 2008, 25, 342.
doi: 10.1039/b705661n pmid: 18389141 |
|
|
(c) Boucard, V.; Broustal, G.; Campagne, J. M. Eur. J. Org. Chem. 2007, 225.
pmid: 18389141 |
|
|
(d) Mondon, M.; Gesson, J.-P. Curr. Org. Synth. 2006, 3, 41.
doi: 10.2174/157017906775473966 pmid: 18389141 |
|
| [45] |
Ren, Q.; Sun, S.; Huang, J.; Li, W.; Wu, M.; Guo, H.; Wang, J.: Chem. Commun. 2014, 50, 6137.
doi: 10.1039/C4CC01736F |
| [46] |
Clerici, P.; Wennemers, H. Org. Biomol. Chem. 2012, 10, 110.
doi: 10.1039/c1ob06638b pmid: 22068641 |
| [47] |
Kolarovic, A.; Käslin, A.; Wennemers, H. Org. Lett. 2014, 16, 4236.
doi: 10.1021/ol501936n pmid: 25100030 |
| [48] |
Ling, T.; Rivas, F. Tetrahedron 2016, 72, 6729.
doi: 10.1016/j.tet.2016.09.002 |
| [49] |
(a) Corey, E. J.; Guzman-Perez, A. Angew. Chem., Int. Ed. 1998, 37, 388.
doi: 10.1002/(SICI)1521-3773(19980302)37:4【-逻*辑*与-】#x00026;lt;388::AID-ANIE388【-逻*辑*与-】#x00026;gt;3.0.CO;2-V |
|
(b) Quasdorf, K. W.; Overman, L. E. Nature 2014, 516, 181.
doi: 10.1038/nature14007 |
|
|
(c) Liu, Y.; Han, S. J.; Liu, W. B.; Stoltz, B. M. Acc. Chem. Res. 2015, 48, 740.
doi: 10.1021/ar5004658 |
|
|
(d) Li, C.; Ragab, S. S.; Liu, G.; Tang, W. Nat. Prod. Rep. 2020, 37, 276.
doi: 10.1039/C9NP00039A |
|
| [50] |
(a) Arakawa, Y.; Fritz, S. P.; Wennemers, H. J. Org. Chem. 2014, 79, 3937.
doi: 10.1021/jo500403q |
|
(b) Musa, M. A.; Cooperwood, J. S.; Khan, M. O. F. Curr. Med. Chem. 2008, 15, 2664.
doi: 10.2174/092986708786242877 |
|
|
(c) Asai, F.; Iinuma, M.; Tanaka, T.; Takenaka, M.; Mizuno, M. Phytochemistry 1992, 31, 2487.
doi: 10.1016/0031-9422(92)83306-J |
|
| [51] |
Sridharan, V.; Suryavanshi, P. A.; Menéndez, J. C. Chem. Rev. 2011, 111, 7157.
doi: 10.1021/cr100307m |
| [52] |
Engl, O. D.; Fritz, S. P.; Käslin, A.; Wennemers, H. Org. Lett. 2014, 16, 5454.
doi: 10.1021/ol502697s |
| [53] |
Cosimi, E.; Saadi, J.; Wennemers, H. Org. Lett. 2016, 18, 6014.
pmid: 27934387 |
| [54] |
(a) He, M.; Uc, G. J.; Bode, J. W. J. Am. Chem. Soc. 2006, 128, 15088.
doi: 10.1021/ja066380r pmid: 22468232 |
|
(b) Kaeobamrung, J.; Mahatthananchai, J.; Zheng, P.; Bode, J. W. J. Am. Chem. Soc. 2010, 132, 8810.
doi: 10.1021/ja103631u pmid: 22468232 |
|
|
(c) Belmessieri, D.; Morrill, L. C.; Simal, C.; Slawin, A. M.; Smith, A. D. J. Am. Chem. Soc. 2011, 133, 2714.
doi: 10.1021/ja109975c pmid: 22468232 |
|
|
(d) Mahatthananchai, J.; Kaeobamrung, J.; Bode, J. W. ACS Catal. 2012, 2, 494.
pmid: 22468232 |
|
|
(e) Morrill, L. C.; Douglas, J.; Lebl, T.; Slawin, A. M.; Fox, D. J.; Smith, A. D. Chem. Sci. 2013, 4, 4146.
doi: 10.1039/c3sc51791h pmid: 22468232 |
|
|
(f) Yao, W.; Dou, X.; Lu, Y. J. Am. Chem. Soc. 2015, 137, 54.
doi: 10.1021/ja5109358 pmid: 22468232 |
|
|
(g) Young, C. M.; Stark, D. G.; West, T. H.; Taylor, J. E.; Smith, A. D. Angew. Chem., Int. Ed. 2016, 55, 14394.
doi: 10.1002/anie.201608046 pmid: 22468232 |
|
|
(h) Zhang, M. L.; Wu, Z. J.; Zhao, J. Q.; Luo, Y.; Xu, X. Y.; Zhang, X. M.; Yuan, W. C. Org. Lett. 2016, 18, 5110.
doi: 10.1021/acs.orglett.6b02558 pmid: 22468232 |
|
|
(i) Xu, D.; Zhang, Y.; Ma, D. Tetrahedron Lett. 2010, 51, 3827.
doi: 10.1016/j.tetlet.2010.05.077 pmid: 22468232 |
|
|
(j) Sinha, D.; Perera, S.; Zhao, J. C. G. Chem. Eur. J. 2013, 19, 6976.
doi: 10.1002/chem.201300168 pmid: 22468232 |
|
| [55] |
Jin, H.; Lee, J.; Shi, H.; Lee, J. Y.; Yoo, E. J.; Song, C. E.; Ryu, D. H. Org. Lett. 2018, 20, 1584.
doi: 10.1021/acs.orglett.8b00331 |
| [56] |
Bahlinger, A.; Fritz, S. P.; Wennemers, H. Angew. Chem., Int. Ed. 2014, 53, 8779.
doi: 10.1002/anie.201310532 pmid: 24644150 |
| [57] |
Cosimi, E.; Engl, O. D.; Wennemers, H. Angew. Chem., Int. Ed. 2016, 55, 13127.
doi: 10.1002/anie.201607146 pmid: 27632946 |
| [58] |
(a) Mitsunuma, H.; Shibasaki, M.; Kanai, M. Angew. Chem., Int. Ed. 2012, 51, 5217.
doi: 10.1002/anie.201201132 pmid: 20063336 |
|
(b) Shimizu, Y.; Kanai, M.; Shibasaki, M. Angew. Chem., Int. Ed. 2010, 49, 1103.
doi: 10.1002/anie.200906678 pmid: 20063336 |
|
|
(c) Overman, L. E.; Paone, D. V.; Stearns, B. A. J. Am. Chem. Soc. 1999, 121, 7702.
doi: 10.1021/ja991714g pmid: 20063336 |
|
|
(d) Lemieux, R. M.; Meyers, A. I. J. Am. Chem. Soc. 1998, 120, 5453.
doi: 10.1021/ja9804159 pmid: 20063336 |
|
| [59] |
Liu, T.; Liu, W.; Shao, Z. J. Org. Chem. 2015, 80, 4950.
doi: 10.1021/acs.joc.5b00302 |
| [60] |
Engl, D. O.; Fritz, S. P.; Wennemers, H. Angew. Chem., Int. Ed. 2015, 54, 8193.
doi: 10.1002/anie.201502976 |
| [61] |
(a) Trost, B. M.; Chung, C. K.; Pinkerton, A. B. Angew. Chem., Int. Ed. 2004, 43, 4327.
doi: 10.1002/anie.200460058 pmid: 16551097 |
|
(b) Fleming, J. J.; Du Bois, J. J. Am. Chem. Soc. 2006, 128, 3926.
pmid: 16551097 |
|
| [62] |
Wang, Y.; Mo, M.; Zhu, K.; Zheng, C.; Zhang, H.; Wang, W.; Shao, Z. Nat. Commun. 2015, 6, 8544.
doi: 10.1038/ncomms9544 |
| [63] |
Ye, W.; Jiang, Z.; Zhao, Y.; Goh, S. L. M.; Leow, D.; Soh, Y.-T.; Tan, C.-H. Adv. Synth. Catal. 2007, 349, 2454.
doi: 10.1002/adsc.200700326 |
| [64] |
Jiang, Z.; Yang, Y.; Pan, Y.; Zhao, Y.; Liu, H.; Tan, C.-H. Chem. Eur. J. 2009, 15, 4925.
doi: 10.1002/chem.200802601 |
| [65] |
Xu, J.; Fu, X.; Low, R.; Goh, Y.-P.; Jiang, Z.; Tan, C.-H. Chem. Commun. 2008, 5526.
|
| [66] |
(a) Okino, T.; Hoashi, Y.; Takemoto, Y. J. Am. Chem. Soc. 2003, 125, 12672.
doi: 10.1021/ja036972z |
|
(b) Okino, T.; Hoashi, Y.; Furukawa, T.; Xu, X.; Takemoto, Y. J. Am. Chem. Soc. 2005, 127, 119.
doi: 10.1021/ja044370p |
|
| [67] |
Jin, H.; Kim, S. T.; Hwang, G.-S.; Ryu, D. H. J. Org. Chem. 2016, 81, 3263.
doi: 10.1021/acs.joc.6b00218 |
| [68] |
(a) Sim, J. H.; Song, C. E. Angew. Chem., Int. Ed. 2017, 56, 1835.
doi: 10.1002/anie.201611466 pmid: 34163692 |
|
(b) Klijn, J. E.; Engberts, J. B. F. N. Nature 2005, 435, 746.
doi: 10.1038/435746a pmid: 34163692 |
|
|
(c) Jung, Y.; Marcus, R. A. J. Am. Chem. Soc. 2007, 129, 5492.
doi: 10.1021/ja068120f pmid: 34163692 |
|
|
(d) Guo, W.; Liu, X.; Liu, Y.; Li, C. ACS Catal. 2018, 8, 328.
doi: 10.1021/acscatal.7b02118 pmid: 34163692 |
|
|
(e) Kitanosono, T.; Kobayashi, S. Chem. Eur. J. 2020, 26, 9408.
doi: 10.1002/chem.201905482 pmid: 34163692 |
|
|
(f) Cortes-Clerget, M.; Yu, J.; Kincaid, J. R. I.; Walde, P.; Gallou, F.; Lipshutz, B. H. Chem. Sci. 2021, 12, 4237.
doi: 10.1039/d0sc06000c pmid: 34163692 |
|
| [69] |
Sim, J. H.; Park, J. H.; Maity, P.; Song, C. E. Org. Lett. 2019, 21, 6715.
doi: 10.1021/acs.orglett.9b02320 |
| [70] |
(a) Akiyama, T.; Itoh, J.; Yokota, K.; Fuchibe, K. Angew. Chem., Int. Ed. 2004, 43, 1566.
doi: 10.1002/anie.200353240 pmid: 15113196 |
|
(b) Uraguchi, D.; Terada, M. J. Am. Chem. Soc. 2004, 126, 5356.
pmid: 15113196 |
|
|
(c) Wu, X.; Li, M.; Gong, L. Acta Chim. Sinica 2013, 71, 1091. (in Chinese)
doi: 10.6023/A13030279 pmid: 15113196 |
|
|
( 吴祥, 李明丽, 龚流柱, 化学学报 2013, 71, 1091.)
doi: 10.6023/A13030279 pmid: 15113196 |
|
| [71] |
Hatano, M.; Moriyama, K.; Maki, T.; Ishihara, K. Angew. Chem., Int. Ed. 2010, 49, 3823.
doi: 10.1002/anie.201000824 |
| [72] |
Song, J.; Shih, H.-W.; Deng, L. Org. Lett. 2007, 9, 603.
pmid: 17256945 |
| [73] |
Bae, H. Y.; Kim, M. J.; Sim, J. H.; Song, C. E. Angew. Chem., Int. Ed. 2016, 55, 10825.
doi: 10.1002/anie.201605167 |
| [74] |
Gulevich, A. V.; Zhdanko, A. G.; Orru, R. V. A.; Nenajgenko, V. G. Chem. Rev. 2010, 110, 5235.
doi: 10.1021/cr900411f pmid: 20608735 |
| [75] |
Odriozola, A.; Oiarbide, M.; Palomo, C. Chem. Eur. J. 2017, 23, 12758.
doi: 10.1002/chem.201703526 |
| [76] |
Oh, J.-S.; Lee, J.-W.; Ryu, T. H.; Lee, J. H.; Song, C. E. Org. Biomol. Chem. 2012, 10, 1052.
doi: 10.1039/C1OB06629C |
| [77] |
Yoshida, Y.; Kasuya, R.; Mino, T.; Sakamoto, M. Org. Biomol. Chem. 2021, 19, 6402.
doi: 10.1039/D1OB00829C |
| [78] |
(a) Nishimura, K.; Ono, M.; Nagaoka, Y.; Tomioka, K. J. Am. Chem. Soc. 1997, 119, 12974.
doi: 10.1021/ja9729950 pmid: 23772965 |
|
(b) Nishimura, K.; Tomioka, K. J. Org. Chem. 2002, 67, 431.
pmid: 23772965 |
|
|
(c) Dong, X.-Q.; Fang, X.; Wang, C.-J. Org. Lett. 2011, 13, 4426.
doi: 10.1021/ol201766k pmid: 23772965 |
|
|
(d) Fang, X.; Li, J.; Wang, C.-J. Org. Lett. 2013, 15, 3448.
doi: 10.1021/ol4015305 pmid: 23772965 |
|
| [79] |
Rigby, C. L.; Dixon, D. J. Chem. Commun. 2008, 39, 3798.
|
| [80] |
Destro, D.; Bottinelli, C.; Ferrari, L.; Albanese, D. C. M.; Bencivenni, G.; Gillick-Healy, M. W.; Adamo, M. F. A. J. Org. Chem. 2020, 85, 5183.
doi: 10.1021/acs.joc.9b03216 pmid: 32053380 |
| [81] |
Maddocks, C. J.; Ermanis, K.; Clarke, P. A. Org. Lett. 2020, 22, 8116.
doi: 10.1021/acs.orglett.0c03090 |
| [82] |
Fukata, Y.; Okamura, T.; Asano, K.; Matsubara, S. Org. Lett. 2014, 16, 2184.
doi: 10.1021/ol500637x |
| [83] |
Ahlemeyer, N. A.; Birman, V. B. Org. Lett. 2016, 18, 3454.
doi: 10.1021/acs.orglett.6b01639 pmid: 27384085 |
| [84] |
Ahlemeyer, N. A.; Streff, E. V.; Muthupandi, P.; Birman, V. B. Org. Lett. 2017, 19, 6486.
doi: 10.1021/acs.orglett.7b03044 pmid: 29200308 |
| [85] |
Lu, H.; Zhang, J.-L.; Liu, J.-Y.; Li, H.-Y.; Xu, P.-F. ACS Catal. 2017, 7, 7797.
doi: 10.1021/acscatal.7b02651 |
| [86] |
(a) Das, J. P.; Marek, I. Chem. Commun. 2011, 47, 4593.
doi: 10.1039/c0cc05222a |
|
(b) Minko, Y.; Pasco, M.; Lercher, L.; Botoshansky, M.; Marek, I. Nature 2012, 490, 522.
doi: 10.1038/nature11569 |
|
| [87] |
Giacalone, F.; Gruttaduria, M.; Agrigento, P.; Noto, R. Chem. Soc. Rev. 2012, 41, 2406.
doi: 10.1039/C1CS15206H |
| [1] | Chen Yougen, Ding Yuansheng. Recent Progress of Organocatalyzed Group Transfer Polymerization [J]. Acta Chimica Sinica, 2020, 78(8): 733-745. |
| [2] | Cai Qian, Ma Haowen. Recent Advances of Chiral Hypervalent Iodine Reagents [J]. Acta Chim. Sinica, 2019, 77(3): 213-230. |
| [3] | Lu Hong, Liu Jin-Yu, Li Hong-Yu, Xu Peng-Fei. Recent Developments in N-Heterocyclic Carbene and Transition-Metal Cooperative Catalysis [J]. Acta Chim. Sinica, 2018, 76(11): 831-837. |
| [4] | Zhang Mao-Mao, Luo Yuan-Yuan, Lu Liang-Qiu, Xiao Wen-Jing. Advances on Asymmetric Allylic Substitutions under Synergetic Catalysis System with Transition Metals and Organocatalysts [J]. Acta Chim. Sinica, 2018, 76(11): 838-849. |
| [5] | Shi Minglin, Zhan Gu, Du Wei, Chen Yingchun. Direct Asymmetric Aza-Vinylogous Mannich Reaction of Nitrones from Isatins and Ketimines [J]. Acta Chim. Sinica, 2017, 75(10): 998-1002. |
| [6] | Zhang Qichao, Lü Jian, Luo Sanzhong. Carbocation Lewis Acid Catalyzed Redox-Neutral α-C(sp3)H Arylation of Amines [J]. Acta Chim. Sinica, 2016, 74(1): 61-66. |
| [7] | Yu Tianyang, Wang Yao, Xu Pengfei. A New Approach for Supramolecular Iminium Catalysis [J]. Acta Chimica Sinica, 2014, 72(7): 845-848. |
| [8] | Ma Shixiong, Zhong Yuan, Wang Shoulei, Xu Zhaoqing, Chang Min, Wang Rui. Organocatalyzed Asymmetric Allylic Alkylation of MBH-Carbonates with Pyrazolones [J]. Acta Chimica Sinica, 2014, 72(7): 825-829. |
| [9] | Wang Yuhui, Cao Zhongyan, Niu Yanfei, Zhao Xiaoli, Zhou Jian. Highly Enantioselective Organocatalytic aza-Henry Reaction of Nitroalkanes to N-Boc Isatin Ketimines [J]. Acta Chimica Sinica, 2014, 72(7): 867-872. |
| [10] | Sun Xunhao, Peng Jing, Zhang Shuyang, Zhou Qingqing, Dong Lin, Chen Yingchun. Asymmetric Allylic Alkylation of Cyclic N-Sulfonylimines with Morita-Baylis-Hillman Carbonates of Isatins [J]. Acta Chimica Sinica, 2012, 70(16): 1682-1685. |
| [11] | WANG Jian-Ping, CHENG Xi-Xing2, CHEN Qing-Hua*,1. Synthesis and Structure of Some Functionalized Chiral Aziridine Derivatives [J]. Acta Chimica Sinica, 2006, 64(7): 686-690. |
| [12] | GUO Jin-Bo; ZHANG Xi-Yun2; CHEN Qing-Hua*,1,2. Study of the Synthetic Method of Cyclopropane Derivatives Based on 3-Bromo-2,5-dihydro-5-menthyloxyfuran-2-one: Asymmetric Tandem Reaction Starting by Carbon Nucleophile [J]. Acta Chimica Sinica, 2006, 64(19): 2008-2014. |
| [13] | FU Li-Hong*1,2,CHENG Jing-Qiu2,LAI Guo-Li. Biomimetic Synthesis of Calcium Carbonate with the Existence of the Gelatin Matrix [J]. Acta Chimica Sinica, 2005, 63(17): 1626-1632. |
| [14] | LIU Hua-Jie, WU Qing-Sheng, DING Ya-Ping, LIU Lu. Biomimetic Synthesis of Metastable PbCrO4 Nanoparticles by EmulsionLiquid Membrane System with Carrier and Coupled Treatment of Pb(Ⅱ) and Cr(VI) Wastewaters [J]. Acta Chimica Sinica, 2004, 62(10): 946-950. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||