Acta Chimica Sinica ›› 2024, Vol. 82 ›› Issue (8): 833-842.DOI: 10.6023/A24050160 Previous Articles Next Articles
Article
郑坤贵a, 刘君珂a, 胡轶旸a, 尹祖伟a, 周尧a,*( ), 李君涛a, 孙世刚b
), 李君涛a, 孙世刚b
投稿日期:2024-05-16
发布日期:2024-07-01
基金资助:
Kungui Zhenga, Junke Liua, Yiyang Hua, Zuwei Yina, Yao Zhoua,*( ), Juntao Lia, Shigang Sunb
), Juntao Lia, Shigang Sunb
Received:2024-05-16
Published:2024-07-01
Contact:
* E-mail: Supported by:Share
Kungui Zheng, Junke Liu, Yiyang Hu, Zuwei Yin, Yao Zhou, Juntao Li, Shigang Sun. A Lithium Polyacrylate-based High-performance Composite Binder for Graphite Anode[J]. Acta Chimica Sinica, 2024, 82(8): 833-842.
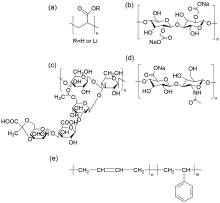
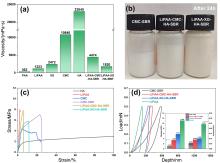

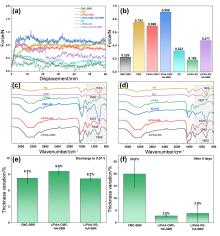

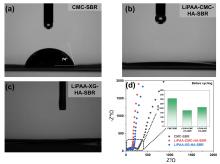
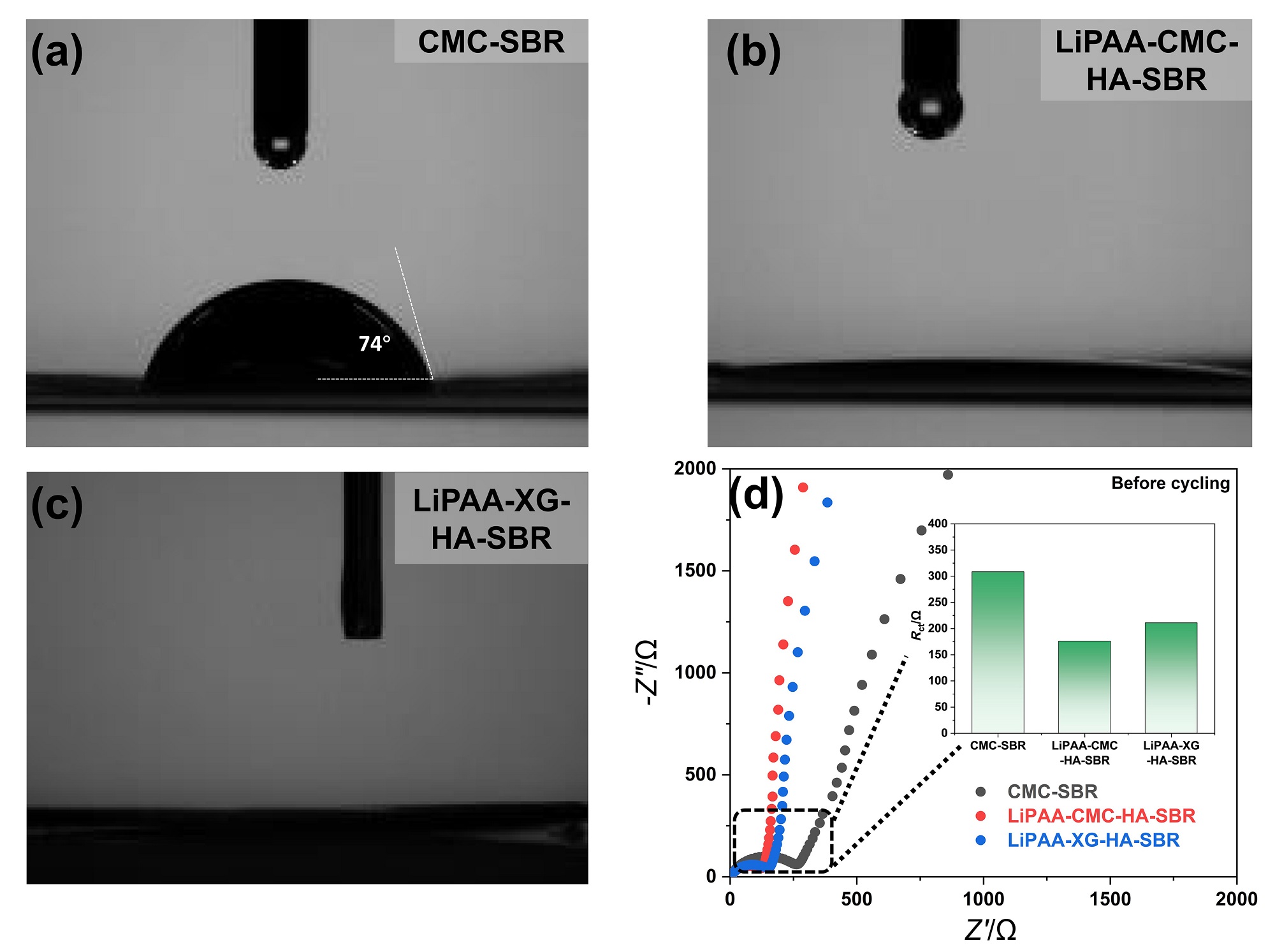




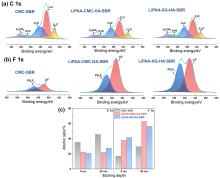

| [1] |
Boudet, H. S. Nat. Energy 2019, 4, 446.
|
| [2] |
Wang, X.; Li, Y. B.; Du, L. Y.; Gao, F. J.; Wu, Q.; Yang, L. J.; Chen, Q.; Wang, X. Z.; Hu, Z. Acta Chim. Sinica 2018, 76, 627 (in Chinese).
doi: 10.6023/A18040135 |
|
(王啸, 李有彬, 杜玲玉, 高福杰, 吴强, 杨立军, 陈强, 王喜章, 胡征, 化学学报, 2018, 76, 627.)
doi: 10.6023/A18040135 |
|
| [3] |
Wu, M. Y.; Xiao, X. C.; Vukmirovic, N.; Xun, S. D.; Das, P. K.; Song, X. Y.; Olalde-Velasco, P.; Wang, D. D.; Weber, A. Z.; Wang, L. W.; Battaglia, V. S.; Yang, W. L.; Liu, G. J. Am. Chem. Soc. 2013, 135, 12048.
|
| [4] |
Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. Science 2011, 334, 75.
doi: 10.1126/science.1209150 pmid: 21903777 |
| [5] |
Agubra, V. A.; Fergus, J. W. J. Power Sources 2014, 268, 153.
|
| [6] |
Xu, T.; Sun, W.; Kong, T. C.; Zhou, J.; Qian, Y. T. Acta Phys.-Chim. Sin. 2024, 40, 85 (in Chinese).
|
|
(许涛, 孙伟, 孔天赐, 周杰, 钱逸泰, 物理化学学报, 2024, 40, 85.)
|
|
| [7] |
Tang, S. Y.; Lu, G. T.; Su, Y.; Wang, G.; Li, X. Z.; Zhang, G. Q.; Wei, Y.; Zhang, Y. G. Acta Phys.-Chim. Sinica 2022, 38, 33 (in Chinese).
|
|
(唐诗怡, 鹿高甜, 苏毅, 王广, 李炫璋, 张广琦, 魏洋, 张跃钢, 物理化学学报, 2022, 38, 33.)
|
|
| [8] |
Park, T. S.; Oh, E. S.; Lee, S. M. J. Power Sources 2014, 248, 1191.
|
| [9] |
Hofmann, K.; Hegde, A. D.; Liu-Theato, X.; Gordon, R.; Smith, A.; Willenbacher, N. J. Power Sources 2024, 593, 233996.
|
| [10] |
Wang, X. Y.; Zhang, Y.; Ma, L.; Wei, L. M. Acta Chim. Sinica 2019, 77, 24 (in Chinese).
|
|
(王晓钰, 张渝, 马磊, 魏良明, 化学学报, 2019, 77, 24.)
doi: 10.6023/A18070272 |
|
| [11] |
Lee, J. H.; Paik, U.; Hackley, V. A.; Choi, Y. M. J. Power Sources 2006, 161, 612.
|
| [12] |
Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C. F.; Fuller, T. F.; Luzinov, I.; Yushin, G. ACS Appl. Mater. Interfaces 2010, 2, 3004.
|
| [13] |
Li, Z. H.; Tang, W. T.; Yang, Y. J.; Lai, G. Y.; Lin, Z.; Xiao, H. Y.; Qiu, J. C.; Wei, X. J.; Wu, S. X.; Lin, Z. Adv. Funct. Mater. 2022, 32, 2206615.
|
| [14] |
Dang, D. Y.; Wang, Y. K.; Wang, M.; Hu, J. Z.; Ban, C. M.; Cheng, Y. T. ACS Appl. Energy Mater. 2020, 3, 10940.
|
| [15] |
Guo, M. J.; Xiang, C. C.; Hu, Y. Y.; Deng, L.; Pan, S. Y.; Lv, C.; Chen, S. X.; Deng, H. T.; Sun, C. D.; Li, J. T.; Zhou, Y.; Sun, S. G. Electrochim. Acta 2022, 425, 140704.
|
| [16] |
Wei, L. M.; Chen, C. X.; Hou, Z. Y.; Wei, H. Sci. Rep. 2016, 6, 19583.
|
| [17] |
Luo, C.; Wu, X. F.; Zhang, T.; Chi, S. S.; Liu, Z. Y.; Wang, J.; Wang, C. Y.; Deng, Y. H. Macromol. Mater. Eng. 2020, 306, 2000525.
|
| [18] |
Lee, H. A.; Shin, M. Y.; Kim, J. M.; Choi, J. W.; Lee, H. S. Adv. Mater. 2021, 33, e2007460.
|
| [19] |
Li, Y.; Jin, B. Y.; Wang, K. Y.; Song, L. N.; Ren, L. H.; Hou, Y.; Gao, X.; Zhan, X. L.; Zhang, Q. H. Chem. Eng. J. 2022, 429, 132235.
|
| [20] |
Hu, L. L.; Jin, M. H.; Zhang, Z.; Chen, H. X.; Ajdari, F. B.; Song, J. X. Adv. Funct. Mater. 2022, 32, 2111560.
|
| [21] |
Wang, R.; Liu, Z. K.; Yan, C.; Jia, L.; Huang, Y. H. Acta Phys.-Chim. Sin. 2023, 39, 81 (in Chinese).
|
|
(汪茹, 刘志康, 严超, 伽龙, 黄云辉, 物理化学学报, 2023, 39, 81.)
|
|
| [22] |
Pieczonka, N. P. W.; Borgel, V.; Ziv, B.; Leifer, N.; Dargel, V.; Aurbach, D.; Kim, J. H.; Liu, Z. Y.; Huang, X. S.; Krachkovskiy, S. A.; Goward, G. R.; Halalay, I.; Powell, B. R.; Manthiram, A. Adv. Energy Mater. 2015, 5, 1501008.
|
| [23] |
Wang, Y. Q.; Ma, Z.; Cao, Z.; Cai, T.; Liu, G.; Cheng, H. R.; Zhao, F.; Cavallo, L.; Li, Q.; Ming, J. Adv. Funct. Mater. 2023, 33, 2305974.
|
| [24] |
Lee, Y. S.; Ryu, K. S. Sci. Rep. 2017, 7, 16617.
|
| [25] |
Rahim, S.; Naveed, A.; Amir, A. R.; Yang, C.; Chen, Y. J.; Hu, J. P.; Zhao, X. H.; Peng, Y.; Deng, Z. Acta Phys.-Chim. Sin. 2019, 35, 1382 (in Chinese).
|
|
(拉希姆•沙阿, 纳维德•阿拉姆, 阿米尔•拉扎克, 杨成, 陈宇杰, 胡加鹏, 赵晓辉, 彭扬, 邓昭, 物理化学学报, 2019, 35, 1382.)
|
|
| [26] |
Weisenberger, C.; Harrison, D. K.; Zhou, C. K.; Knoblauch, V. Electrochim. Acta 2023, 461, 142629.
|
| [27] |
Pan, S. Y.; Yang, X. R.; Zhou, Y.; Lv, C.; Deng, H, T.; Guo, M. J.; Chen, S. X.; Hu, Y. Y.; Deng, L.; Qiao, Y.; Li, J. T.; Huang, L.; Yang, Y.; Sun, S. G. ACS Appl. Mater. Interfaces 2021, 13, 55700.
|
| [28] |
Huang, Y. S.; Wang, C. A.; Lv, H. F.; Xie, Y. S.; Zhou, S. Y.; Ye, Y. D.; Zhou, E.; Zhu, T. Y.; Xie, H. Y.; Jiang, W.; Wu, X. J.; Kong, X. H.; Jin, H. C.; Ji, H. X. Adv. Mater. 2024, 36, 2308675.
|
| [29] |
Yang, Y. Z.; Wang, J.; Li, Z. L.; Yang, Z.; Wang, B.; Zhao, H. L. ACS Nano 2024, 18, 7666.
|
| [1] | Xiaoyu Gu, Jin Li, Qian Sun, Chaoyang Wang. Microcalorimetry Analysis of Thermal Runaway Process in Lithium-ion Batteries [J]. Acta Chimica Sinica, 2024, 82(2): 146-151. |
| [2] | Jia Yanggang, Chen Shijie, Shao Xia, Cheng Jie, Lin Na, Fang Daolai, Mao Aiqin, Li Canhua. Preparation and High-performance Lithium-ion Storage of Cobalt-free Perovskite High-entropy Oxide Anode Materials [J]. Acta Chimica Sinica, 2023, 81(5): 486-495. |
| [3] | Yalan Zhang, Zhixiang Yuan, Hao Zhang, Jianjun Zhang, Guanglei Cui. Research Progress of High-energy-density Solid-state Lithium Ion Batteries Employing Ni-rich Ternary Cathodes [J]. Acta Chimica Sinica, 2023, 81(12): 1724-1738. |
| [4] | Wanying Chang, Yingying Tan, Jingyi Wu, Yingjie Liu, Jinhai Cai, Chunyan Lai. Study on the Properties of Polyethylene Oxide Based Solid State Electrolyte Enhanced by Three-Dimensional Structured Li6.28La3Zr2Al0.24O12 [J]. Acta Chimica Sinica, 2023, 81(12): 1708-1715. |
| [5] | Shuang Zhang, Chengfei Yang, Yubo Yang, Ningning Feng, Gang Yang. Electrochemical Behaviors of LixMO (x=0.79, 0.30, 0.08; M=Ni/Co/Mn) Recycled from Spent Li-ion Batteries as Cathodic Catalyst for Lithium-Oxygen Battery [J]. Acta Chimica Sinica, 2022, 80(9): 1269-1276. |
| [6] | Shouxiao Chen, Junke Liu, Weichen Zheng, Guozhen Wei, Yao Zhou, Juntao Li. Electron/ion Conductor Double-coated LiNi0.8Co0.1Mn0.1O2 Li-ion Battery Cathode Material and Its Electrochemical Performance [J]. Acta Chimica Sinica, 2022, 80(4): 485-493. |
| [7] | Kai Qiu, Mingxia Yan, Shouwang Zhao, Shengli An, Wei Wang, Guixiao Jia. Theoretical Study on the Structural Stability and Oxygen Ion Oxidation of Al-doped Lithium-ion Battery Layered Cathode Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2 [J]. Acta Chimica Sinica, 2021, 79(9): 1146-1153. |
| [8] | Ping Xu, Xihua Zhang, En Ma, Fu Rao, Chunwei Liu, Peifan Yao, Zhi Sun, Jingwei Wang. Selective Recovery of Lithium from Spent Lithium-ion Batteries Synergized by Carbon and Sulfur Elements [J]. Acta Chimica Sinica, 2021, 79(8): 1073-1081. |
| [9] | Zhi Chang, Yu Qiao, Huijun Yang, Han Deng, Xingyu Zhu, Ping He, Haoshen Zhou. Applications of Metal-organic Frameworks (MOFs) Materials in Lithium-ion Battery/Lithium-metal Battery Electrolytes [J]. Acta Chimica Sinica, 2021, 79(2): 139-145. |
| [10] | Wang Shan, Fan Xiaoyong, Cui Yu, Gou Lei, Wang Xingang, Li Donglin. Three-dimensional Porous Current Collector for Lithium Storage Enhancement of NiO Electrode [J]. Acta Chim. Sinica, 2019, 77(6): 551-558. |
| [11] | Deng Bangwei, Sun Daming, Wan Qi, Wang Hao, Chen Tao, Li Xuan, Qu Meizhen, Peng Gongchang. Review of Electrolyte Additives for Ternary Cathode Lithium-ion Battery [J]. Acta Chim. Sinica, 2018, 76(4): 259-277. |
| [12] | He Qian, Zhang Chong, Li Xiao, Wang Xue, Mu Pan, Jiang Jiaxing. Pyrene-Based Conjugated Microporous Polymer as High Performance Electrode for Lithium-Ion Batteries [J]. Acta Chim. Sinica, 2018, 76(3): 202-208. |
| [13] | Li Zhiwei, Zhong Jialiang, Chen Nannan, Xue Bing, Mi Hongyu. Template-Assisted Preparation and Lithium Storage Performance of Nitrogen Doped Porous Carbon Sheets [J]. Acta Chim. Sinica, 2018, 76(3): 209-214. |
| [14] | Zheng Zhuo, Wu Zhenguo, Xiang Wei, Guo Xiaodong. Preparation and Electrochemical Performance of High Rate Spherical Layered LiNi0.5Co0.2Mn0.3O2 Cathode Material for Lithium-Ion Batteries [J]. Acta Chim. Sinica, 2017, 75(5): 501-507. |
| [15] | Zhang Guobin, Xiong Tengfei, Pan Xuelei, Yan Mengyu, Han Chunhua, Mai Liqiang. In Situ Observation and Mechanism Investigation of Lattice Breathing in Vanadium Oxide Cathode [J]. Acta Chim. Sinica, 2016, 74(7): 582-586. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||