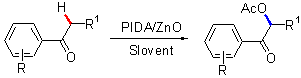| [1] |
赵瑜, 段玉荣, 史时辉, 白育斌, 黄亮珠, 杨晓军, 张琰图, 冯彬, 张建波, 张秋禹. 可见光促进高价碘(III)试剂参与反应的研究进展[J]. 有机化学, 2023, 43(12): 4106-4140. |
| [2] |
马豪杰, 周风院, 苏凡文, 韩波, 李然, 张玉琦, 王记江. 碘促进N,N-二甲基乙酰胺(DMA)与胺的转酰胺反应[J]. 有机化学, 2023, 43(11): 3960-3965. |
| [3] |
顾依钰, 吕晓庆, 马晓东, 张浩健, 嵇媛媛, 丁婉婧, 沈立. 新型吡啶苯磺酰胺联喹啉基异羟肟酸类PI3K/HDAC双靶点抑制剂的设计、合成和生物活性研究[J]. 有机化学, 2020, 40(1): 95-107. |
| [4] |
刘天宝, 彭艳芬, 桂美芳, 章敏. 无金属催化快速合成2-芳酰氨基萘并[1, 2-d]噻唑[J]. 有机化学, 2019, 39(11): 3199-3206. |
| [5] |
张洁雨, 柯求敏, 陈家英, 何平, 严国兵. 芳基三氟甲基酮类化合物的合成进展[J]. 有机化学, 2019, 39(1): 74-83. |
| [6] |
刘天宝, 彭艳芬, 王雅洁, 雍家远, 汪新. 无溶剂研磨法合成硫代氨基胍衍生物[J]. 有机化学, 2018, 38(4): 969-974. |
| [7] |
蒋筱莹, 米治胜, 郭冬艳, 谢媛媛, 金婷婷, 王文慧. 一种以硒脲为原料高效合成5-氨基四氮唑类化合物的新方法[J]. 有机化学, 2016, 36(9): 2150-2156. |
| [8] |
高文涛, 赵鹏波, 赵宾宾, 李阳. 卤素功能化靛红的合成及其烃基化、还原和酰基化反应研究[J]. 有机化学, 2014, 34(1): 126-136. |
| [9] |
赵育, 倪春燕, 张虞婷, 朱俐. 山荷叶素异羟肟酸和硫醇衍生物的合成与抑制肿瘤细胞增殖活性[J]. 有机化学, 2013, 33(01): 169-173. |
| [10] |
李晓晖, 黄大卫, 孙蕾, 修志龙, 西野宪和. 基于Chlamydocin骨架设计合成环肽类HDACi及其抗肿瘤活性[J]. 有机化学, 2011, 31(03): 312-316. |
| [11] |
田敉, 王孝科. 二醋酸碘苯促进苯并咪唑类化合物的合成[J]. 有机化学, 2009, 29(10): 1654-1658. |
| [12] |
李英俊* ; 刘 军 ; 许永廷 ; 靳 焜 ;
孙淑琴 ; 丁万刚; 刘丽军
. N,N-二取代甘氨酸四乙酰基葡萄糖酯的合成与表征[J]. 有机化学, 2009, 29(02): 302-306. |
| [13] |
王道林*; 韩 珊; 谷 峥 ; 徐 姣. 1-(2-苯并呋喃酰基)愈创兰烃薁的合成[J]. 有机化学, 2008, 28(9): 1641-1645. |
| [14] |
周其忠,何春林,陈振初. 二醋酸碘苯在碘和三苯基膦作用下酰化醇、酚和胺[J]. 有机化学, 2008, 28(06): 1097-1101. |
| [15] |
李家明,汪志勇,曾雷,周明明. 一步法合成N-1,3-二羟基丙烷-2-基烷基乙酰胺[J]. 有机化学, 2006, 26(07): 971-974. |