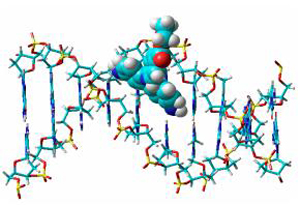
| [1] Dalton, L. R.; Sapochak, L. S. J. Phys. Chem. 1993, 97, 2871.[2] Galabrase, X. J.; Chem, L. T.; Green, J. G. J. Am. Chem. Soc. 1991, 113, 227.[3] Zhou, G. Y.; Wang, D.; Wang, X. M.; Xu, X. G.; Shao, Z. S.; Zhao, X.; Jiang, M. H. Opt. Tech. 2002, 28, 372 (in Chinese).(周广勇, 王东, 王筱梅, 许心光, 邵宗书, 赵显, 方奇, 蒋民华, 光学技术, 2002, 28, 372.)[4] Kotle, Z.; Segal, J.; Sigalov, M.; Benaasally, A.; Khodorkobsky, A. Synth. Met. 2000, 115.[5] Han, L. Z.; Wang, Z.; Hua, Y. J.; Ren, A. M.; Liu, Y. L.; Liu, P. J. Acta Chim. Sinica 2012, 70, 579 (in Chinese).(韩立志, 王卓, 华英杰, 任爱民, 刘艳玲, 刘朋军, 化学学报, 2012, 70, 579.)[6] Zhang, P.; Li, C.; Li, Y. W.; Tu, Y. F. J. Chin. J. Chem. 2013, 2013, 31, 1439.[7] Nsib, F.; Ayed, N.; Chevalier, Y. Dye Pigm. 2007, 74, 133.[8] Mishra, A. K.; Jacob, J.; MÜllen, K. Dye Pigm. 2007, 75, 1.[9] Yoon, K. R.; Ko, S. O.; Lee, S. M.; Lee, H. S. Dye Pigm. 2007, 75, 567.[10] Curiel, D.; Cowley, A.; Beer, P. D. Chem. Commun. 2005, 236.[11] Yu, M.; Lin, H.; Lin, H. Supramol. Chem. 2008, 20, 357.[12] Omura, S.; Sasaki, Y.; Iwai, Y.; Takeshima, H. J. Antibiot. 1995, 48, 535.[13] Chen, W. J.; Zhou, C. X.;Yao, P. F.; Wang, X. X.; Tan, J. H.; Li, D.; Ou, T. M.; Gu, L. Q.; Huang, Z. S. Bioorg. Med. Chem. 2012, 20, 2829.[14] Huang, F. C.; Chang, C. C.; Lou, P. J.; Kuo, I. C.; Chien, C. W.; Chen, C. T.; Shieh, F. Y.; Chang, T. C.; Lin, J. J. Mol. Cancer Res. 2008, 6, 955.[15] Jia, T.; Xiang, J.; Wang, J.; Guo, P.; Yu, J. P. Org. Biomol. Chem. 2013, 11, 5512.[16] Yang, P.; Yang, Q.; Qian, X. H. Tetrahedron 2005, 61, 11895.[17] Monchaud, D.; Yang, P.; Lacroix, L.; Teulade-Fichou, M.-P.; Mergny, J.-L. Angew. Chem., Int. Ed. 2008, 47, 4858.[18] House, H. O.; Chu, C. Y.; Wilkins, J. M.; Umen, M. J. Org. Chem. 1975, 40, 1460.[19] Kato, Y.; Conn, M. M.; Rebek, Jr. J. Am. Chem. Soc. 1994, 116, 3279.[20] Freeman, H. S.; Butler, J. R. J. Org. Chem. 1978, 26, 4975.[21] Pasternack, R. F.; Gibbs, E. J.; Villafranca, J. J. Biochemistry 1983, 22, 2406.[22] Pyle, A. M.; Rehmann, J. P.; Meshoyrer, R.; Kumar, C. V.; Turro, N. J.; Barton, J. K. J. Am. Chem. Soc. 1989, 111, 3051.[23] Dumat, B.; Bordeau, G.; Faurel-Paul, E.; Mahuteau-Betzer, F.; Saettel, N.; Bombled, M.; Metgé, G.; Charra, F.; Fiorini-Debuisschert, C.; Teulade-Fichou, M. P. Biochimie 2011, 93, 1209.[24] Bag, S. S.; Ghorai, S.; Pradhan, M. K.; Kundu, R.; Jana, S. Bioorg. Med. Chem. Lett. 2013, 23, 96.[25] Bag, S. S.; Ghorai, S.; Jana, S.; Mukherjee, C. RSC Adv. 2013, 3, 5374.[26] Lepecq, J. B.; Paoletti, C. J. Mol. Biol. 1967, 27, 87.[27] Dunlop, H. G.; Tucker, S. H. J. Chem. Soc. 1939, 1945.[28] Benson, S. C.; Singh, P.; Glazer, A. N. Nucleic Acids Res. 1993, 21, 5727.[29] Demas, J. N.; Crosby, G. A. J. Phys. Chem. 1971, 75, 991.Karstens, T.; Kobs, K. J. Phys. Chem. 1980, 84, 1871. |