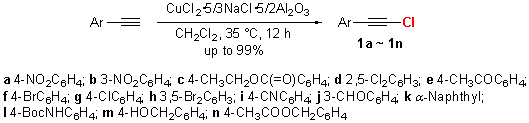| [1] (a) Chen, D.-X.; Yuan, Z.-L.; Cai, H.-T.; Zeng, R.-W.; Kong, L.-C.; Zhu, G.-G. J. Org. Chem. 2011, 76, 4071.
(b) Chen, X.-Y.; Chen, D.-X.; Lu, Z.-H.; Kong, L.-C.; Zhu, G.-G. J. Org. Chem. 2011, 76, 6338.
[2] (a) Nishihara, Y.; Ikegashira, K.; Mori, A.; Hiyama, T. Tetrahedron Lett. 1998, 39, 4075.
(b) Nishihara, Y.; Ikegashira, K.; Hirabayashi, K.; Ando, J.-I.; Mori, A.; Hiyama, T. J. Org. Chem. 2000, 65, 1780.
[3] Poulsen, T. B.; Bernardi, L.; Alemán, J.; Overgaard, J.; Jørgensen, K. A. J. Am. Chem. Soc. 2007, 129, 441.
[4] (a) Villeneuve, K.; Riddell, N.; Jordan, R. W.; Tsui, G. C.; Tam, W. Org. Lett. 2004, 6, 4543.
(b) Liu, P.; Jordan, R. W.; Kibbee, S. P.; Goddard, J. D.; Tam, W. J. Org. Chem. 2006, 71, 3793.
(c) Yao, Z.-K.; Yu, Z.-X. J. Am. Chem. Soc. 2011, 133, 10864.
[5] Cahiez, G.; Gager, O.; Buendia, J. Angew. Chem., Int. Ed. 2010, 49, 1278.
[6] Sud, D.; Wigglesworth, T. J.; Branda, N. R. Angew. Chem., Int. Ed. 2007, 46, 8017.
[7] Martins, M. A. P.; Emmerich, D. J.; Pereira, C. M. P.; Cunico, W.; Rossato, M.; Zanatta, N.; Bonacorso, H. G. Tetrahedron Lett. 2004, 45, 4935.
[8] Sasson, Y.; Webster, O. W. J. Chem. Soc., Chem. Commun. 1992, 1200.
[9] Ballester, M.; Castañer, J.; Riera, J.; Tabernero, I. J. Org. Chem. 1986, 51, 1413.
[10] Braga, A. L.; Cornasset, J. V. Synth. Commun. 1989, 19, 2877.
[11] Hashmi, A. S. K.; Ramamurthi, T. D.; Todd, M. H.; Tsang, A. S.-K.; Graf, K. Aust. J. Chem. 2010, 63, 1619.
[12] Vilhelmsen, M. H.; Andersson, A. S.; Nielsen, M. B. Synthesis. 2009, 1469.
[13] Carran, J.; Waschbüsch, R.; Marinetti, A.; Savignac, P. Synthesis 1996, 1494.
[14] Villieras, J.; Perriot, P.; Normant, J. F. Synthesis 1975, 458.
[15] Uemura, S.; Onoe, A.; Okano, M. J. Chem. Soc., Chem. Commun. 1975, 23, 925.
[16] Uemura, S.; Okazaki, H.; Onoe, A.; Okano, M. J. Chem. Soc., Perkin Trans. 1 1977, 676.
[17] Kodomari, M.; Satoh, H.; Yoshitomi, S. Nippon Kagaku Kaishi 1986, 12, 1813.
[18] Bai, D.-H.; Li, C.-J.; Li, J.; Jia, X.-S. Chin. J. Org. Chem. 2012, 32, 994 (in Chinese).
(白东虎, 李春举, 李健, 贾学顺, 有机化学, 2012, 32, 994.)
[19] Jithunsa, M.; Ueda, M.; Miyata, O. Org. Lett. 2011, 13, 518.
[20] Mao, Z.-F.; Wang, Z.; Xu, Z.-Q.; Huang, F.; Yu, Z.-K.; Wang, R. Org. Lett. 2012, 14, 3854.
[21] Wagner, C. D.; Riggs, W. M.; Davis, L. E.; Moulder, J. F. In Handbook of X-ray Photoelectron Spectroscopy, Eds.: Muilenberg, G. E., Perkin-Elmer Press, New York, 1979, p. 82.
[22] Li, S.-P.; Kuang, C.-X.; Sun, Y.-Z. Chem. World 2010, 52 (in Chinese).
(李世鹏, 匡春香, 孙跃枝, 化学世界, 2010, 52.)
[23] Montheard, J.-P.; Camps, M.; Benzaid, A. Tetrahedron Lett. 1982 |