有机化学 ›› 2021, Vol. 41 ›› Issue (4): 1337-1358.DOI: 10.6023/cjoc202009031 上一篇 下一篇
综述与进展
王宇a, 王泾洋a, 吴啸宇a, 丁广妮b, 张兆国a,*( ), 谢小敏a,*(
), 谢小敏a,*( )
)
收稿日期:2020-09-13
修回日期:2020-10-28
发布日期:2020-11-19
通讯作者:
张兆国, 谢小敏
作者简介:基金资助:
Yu Wanga, Jingyang Wanga, Xiaoyu Wua, Guangni Dingb, Zhaoguo Zhanga,*( ), Xiaomin Xiea,*(
), Xiaomin Xiea,*( )
)
Received:2020-09-13
Revised:2020-10-28
Published:2020-11-19
Contact:
Zhaoguo Zhang, Xiaomin Xie
About author:Supported by:文章分享
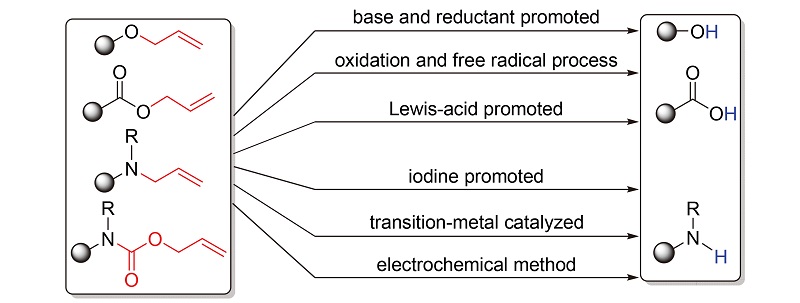
烯丙基是有机合成中常用的保护基团, 具有引入简单, 在酸/碱性及还原剂等条件下稳定, 在相对温和的条件下选择性地脱保护等特点, 在有机合成特别是药物和天然产物的合成研究中具有重要地位. 近几十年来, 研究者们对各类烯丙基的脱保护方法进行了广泛研究. 按碱及还原剂促进、氧化及自由基过程、路易斯酸促进、碘促进、过渡金属催化及电化学方法等分类, 对脱烯丙基保护方法的研究进展进行了综述.
王宇, 王泾洋, 吴啸宇, 丁广妮, 张兆国, 谢小敏. 脱烯丙基反应研究进展[J]. 有机化学, 2021, 41(4): 1337-1358.
Yu Wang, Jingyang Wang, Xiaoyu Wu, Guangni Ding, Zhaoguo Zhang, Xiaomin Xie. Advances in Deallylation[J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1337-1358.



| [1] |
Greene, T.W.; Wuts, P.G. M., Protective Groups in Organic Synthesis, 3rd ed., John Wiley & Sons, Inc., New York, 1999.
|
| [2] |
(a) Weissman, S.A.; Zewge, D. Tetrahedron 2005, 61,7833.
|
|
(b) Tang, J.-Y.; Liu, H.-X.; Huang, C.-S. Technol. Dev. Chem. Ind. 2016, 45,15. (in Chinese)
|
|
|
( 唐剑耀, 刘红星, 黄初升, 化学技术与开发, 2016, 45,15.)
|
|
|
(c) Li, Z.J.; Zhang, S.Q.; Wang, A.B.; Cai, M.S. Acta Chim. Sinica 1998, 56,1128. (in Chinese)
|
|
|
( 李中军, 张三奇, 王安邦, 蔡孟深, 化学学报, 1998, 56,1128.)
|
|
|
(d) Zhou, Y.; Zhang, L.R.; Zhang, L.H. Acta Chim. Sinica 2001, 59,1691. (in Chinese)
|
|
|
( 周英, 张亮仁, 张礼和, 化学学报, 2001, 59,1691.)
|
|
|
(e) Deng, X.; Liu, W.; Li, C.; Zhang, Z.; Wang, X.; Liu, J. Chin. J. Org. Chem. 2011, 31,75. (in Chinese)
|
|
|
( 邓喜玲, 刘卫东, 李超, 张志丽, 王孝伟, 刘俊义, 有机化学, 2011, 31,75.)
|
|
| [3] |
(a) Zhang, L.; Wang, Y.; Yu, J.; Zhang, G.; Cai, X.; Wu, Y.; Wang, L. Tetrahedron Lett. 2013, 54,4019.
|
|
(b) Takagi, K.; Fukuda, H.; Shuto, S.; Otaka, A.; Arisawa, M. Adv. Synth. Catal. 2015, 357,2119.
|
|
|
(c) Bu, X.; Williams, M.; Jo, J.; Koide, K.; Welch, C.J. Chem. Commun. 2017, 53,720.
|
|
| [4] |
Prosser, T.J. J. Am. Chem. Soc. 1961, 83,1701.
|
| [5] |
Price, C.C.; Snyder, W.H. J. Am. Chem. Soc. 1961, 83,1773.
|
| [6] |
(a) Oltvoort, J.J.; Kloosterman, M.; van Boom, J.H. Recl. Trav. Chim. Pays-Bas 1983, 102,501.
|
|
(b) Guibe, F.; M'Leux, Y.S. Tetrahedron Lett. 1981, 22,3591.
|
|
| [7] |
Gevorgyan, V.; Yamamoto, Y. Tetrahedron Lett. 1995, 36,7765.
|
| [8] |
Nicolaou, K.C.; Caulfield, T.J.; Kataoka, H.; Stylianides, N.A. J. Am. Chem. Soc. 1990, 112,3693.
|
| [9] |
Gigg, R.; Warren, C.D. J. Chem. Soc. C 1968,1903.
|
| [10] |
Halkes, K.M.; Slaghek, T.M.; Vermeer, H.J.; Kamerling, J.P.; Vliegenthart, J.F. G. Tetrahedron Lett. 1995, 36,6137.
|
| [11] |
Mereyala, H.B.; Lingannagaru, S.R. Tetrahedron 1997, 53,17501.
|
| [12] |
Cunningham, J.; Gigg, R.; Warren, C.D. Tetrahedron Lett. 1964, 5,1191.
|
| [13] |
(a) Gigg, J.; Gigg, R. J. Chem. Soc. C 1966,82.
|
|
(b) Smith, A.B.; Rivero, R.A.; Hale, K.J.; Vaccaro, H.A. J. Am. Chem. Soc. 1991, 113,2092.
|
|
| [14] |
Yamada, H.; Harada, T.; Takahashi, T. J. Am. Chem. Soc. 1994, 116,7919.
|
| [15] |
Lamberth, C.; Bednarski, M.D. Tetrahedron Lett. 1991, 32,7369.
|
| [16] |
Pirrung, F.O. H.; Rutjes, F. P., J.T.; Hiemstra, H.; Speckamp, W.N. Tetrahedron Lett. 1990, 31,5365.
|
| [17] |
Effenberger, F.; Jäger, J. J. Org. Chem. 1997, 62,3867.
|
| [18] |
Kametani, T.; Huang, S.-P.; Ihara, M.; Fukumoto, K. J. Org. Chem. 1976, 41,2545.
|
| [19] |
Thomas, R.M.; Mohan, G.H.; Iyengar, D.S. Tetrahedron Lett. 1997, 38,4721.
|
| [20] |
Li, C.B.; Ji, X.J.; Zhang, S.M.; Lu, M.; Zhao, Z.X.; Cui, Y.; Xu, Y.L.; Yang, Q.C.; Zhang, W.Q. Chin. Chem. Lett. 2003, 14,459.
|
| [21] |
Pawar, B.V.; Lokhande, P.D. Synth. Commun. 2009, 39,2445.
|
| [22] |
Mann, F.G.; Pragnell, M.J. J. Chem. Soc. 1965,4120.
|
| [23] |
Bailey, W.F.; England, M.D.; Mealy, M.J.; Thongsornkleeb, C.; Teng, L. Org. Lett. 2000, 2,489.
pmid: 10814358 |
| [24] |
Sanz, R.; Martinez, A.; Marcos, C.; Fananas, F.J. Synlett 2008,1957.
|
| [25] |
Alonso, E.; Ramón, D.J.; Yus, M. Tetrahedron 1997, 53,14355.
|
| [26] |
Kariyone, K.; Yazawa, H. Tetrahedron Lett. 1970, 11,2885.
|
| [27] |
Choudary, B.M.; Prasad, A.D.; Swapna, V.; Valli, V.L. K.; Bhuma, V. Tetrahedron 1992, 48,953.
|
| [28] |
Yadav, J.S.; Chandrasekhar, S.; Sumithra, G.; Kache, R. Tetrahedron Lett. 1996, 37,6603.
|
| [29] |
Kumar, P.; Cherian, S.K.; Jain, R.; Show, K. Tetrahedron Lett. 2014, 55,7172.
|
| [30] |
Robles, Diaz, R.; Rodriguez Melgarejo, C.; Plaza Lopez-Espinosa, M.T.; Izquierdo Cubero, I. J. Org. Chem. 1994, 59,7928.
|
| [31] |
Yang, S.G.; Park, M.Y.; Kim, Y.H. Synlett 2002,492.
|
| [32] |
Dahlen, A.; Sundgren, A.; Lahmann, M.; Oscarson, S.; Hilmersson, G. Org. Lett. 2003, 5,4085.
|
| [33] |
Escoubet, S.; Gastaldi, S.; Timokhin, V.I.; Ber-trand, M.P.; Siri, D. J. Am. Chem. Soc. 2004, 126,12343.
|
| [34] |
Perchyonok, V.T.; Ryan, S.J.; Langford, S.J.; Hearn, M.T.; Tuck, K.L. Synlett 2008,1233.
|
| [35] |
Balgotra, S.; Venkateswarlu, V.; Vishwakarma, R.A.; Sawant, S.D. Tetrahedron Lett. 2015, 56,4289.
|
| [36] |
Garbers, C.F.; Steenkamp, J.A.; Visagie, H.E. Tetrahedron Lett. 1975, 16,3753.
|
| [37] |
Bhatt, M.V.; El-Morey, S.S. Synthesis 1982,1048.
|
| [38] |
Akiyama, T.; Hirofuji, H.; Ozaki, S. Tetrahedron Lett. 1991, 32,1321.
|
| [39] |
Sakate, S.S.; Kamble, S.B.; Chikate, R.C.; Rode, C.V. New J. Chem. 2017, 41,4943.
|
| [40] |
Nagaraju, M.; Krishnaiah, A.; Mereyala, H.B. Synth. Commun. 2007, 37,2467.
|
| [41] |
(a) Konda, S.G.; Humne, V.T.; Lokhande, P.D. Green Chem. 2011, 13,2354.
|
|
(b) Humne, V.T.; Hasanzadeh, K.; Lokhande, P.D. Res. Chem. Intermed. 2013, 39,585.
|
|
|
(c) Humne, V.; Lokahnde, P. Synth. Commun. 2014, 44,929.
|
|
| [42] |
Patil, A.M.; Kamble, D.A.; Lokhande, P.D. ChemistrySelect 2017, 2,8418.
|
| [43] |
Satyanarayana, K.; Chidambaram, N.; Chandra-sekaran, S. Synth. Commun. 1989, 19,2159.
|
| [44] |
Kadam, S.M.; Nayak, S.K.; Banerji, A. Tetrahedron Lett. 1992, 33,5129.
|
| [45] |
Talukdar, S.; Nayak, S.K.; Banerji, A. J. Org. Chem. 1998, 63,4925.
|
| [46] |
Lee, J.; Cha, J.K. Tetrahedron Lett. 1996, 37,3663.
|
| [47] |
Ohkubo, M.; Mochizuki, S.; Sano, T.; Kawaguchi, Y.; Okamoto, S. Org. Lett. 2007, 9,773.
|
| [48] |
Rajakumar, P.; Murali, V. Synth. Commun. 2003, 33,3891.
|
| [49] |
Ito, H.; Taguchi, T.; Hanzawa, Y. J. Org. Chem. 1993, 58,774.
|
| [50] |
Corey, E.J.; Suggs, J.W. J. Org. Chem. 1973, 38,3224.
|
| [51] |
(a) Gent, P.A.; Gigg, R. J. Chem. Soc., hem. Commun. 1974,277.
|
|
(b) Gigg, R. J. Chem. Soc., erkin Trans. 1 1980,738.
|
|
| [52] |
Sundberg, R.J.; Hamilton, G.S.; Laurino, J.P. J. Org. Chem. 1988, 53,976.
|
| [53] |
Ziegler, F.E.; Brown, E.G.; Sobolov, S.B. J. Org. Chem. 1990, 55,3691.
|
| [54] |
Zacuto, M.J.; Xu, F. J. Org. Chem. 2007, 72,6298.
|
| [55] |
Boss, R.; Scheffold, R. Angew. Chem., nt. Ed. 1976, 15,558.
|
| [56] |
Mori, M.; Ban, Y. Chem. Pharm. Bull. 1976, 24,1992.
|
| [57] |
Bieg, T.; Szeja, W. J. Carbohydr. Chem. 1985, 4,441.
|
| [58] |
Nakayama, K.; Uoto, K.; Higashi, K.; Soga, T.; Kusama, T. Chem. Pharm. Bull. 1992, 40,1718.
|
| [59] |
Mereyala, H.B.; Guntha, S. Tetrahedron Lett. 1993, 34,6929.
|
| [60] |
Honda, M.; Morita, H.; Nagakura, I. J. Org. Chem. 1997, 62,8932.
|
| [61] |
(a) Ishizaki, M.; Yamada, M.; Watanabe, S.-I.; Hoshino, O.; Nishitani, K.; Hayashida, M.; Tanaka, A.; Hara, H. Tetrahedron 2004, 60,7973.
|
|
(b) Yamada, M.; Watanabe, S.-I.; Hoshino, O.; Ishizaki, M.; Hayashida, M.; Tanaka, A.; Hara, H. Chem. Pharm. Bull. 2003, 51,1220.
|
|
| [62] |
(a) Hata, G.; Takahashi, K.; Miyake, A. J. Chem. Soc., hem. Commun. 1970,1392.
|
|
(b) Takahashi, K.; Miyake, A.; Hata, G. Bull. Chem. Soc. Jpn. 1972, 45,230.
|
|
| [63] |
Jeffrey, P.D.; McCombie, S.W. J. Org. Chem. 1982, 47,587.
|
| [64] |
Kunz, H.; Unverzagt, C. Angew. Chem., nt. Ed. 1984, 23,436.
|
| [65] |
Minami, I.; Ohashi, Y.; Shimizu, I.; Tsuji, J. Tetrahedron Lett. 1985, 26,2449.
|
| [66] |
(a) Four, P.; Guibe, F. Tetrahedron Lett. 1982, 23,1825.
|
|
(b) Dangles, O.; Guibé, F.; Balavoine, G.; Lavielle, S.; Marquet, A. J. Org. Chem. 1987, 52,4984.
|
|
| [67] |
Roos, E.C.; Bernabe, P.; Hiemstra, H.; Speckamp, W.N.; Kaptein, B.; Boesten, W.H. J. J. Org. Chem. 1995, 60,1733.
|
| [68] |
Deziel, R. Tetrahedron Lett. 1987, 28,4371.
|
| [69] |
Yamada, T.; Goto, K.; Mitsuda, Y.; Tsuji, J. Tetrahedron Lett. 1987, 28,4557.
|
| [70] |
Garro-Helion, F.; Merzouk, A.; Guibé, F. J. Org. Chem. 1993, 58,6109.
|
| [71] |
(a) Beugelmans, R.; Bourdet, S.; Bigot, A.; Zhu, J. Tetrahedron Lett. 1994, 35,4349.
|
|
(b) Beugelmans, R.; Neuville, L.; Bois-Choussy, M.; Chastanet, J.; Zhu, J. Tetrahedron Lett. 1995, 36,3129.
|
|
| [72] |
Lemaire-Audoire, S.; Savignac, M.; Genêt, J.P.; Bernard, J.-M. Tetrahedron Lett. 1995, 36,1267.
|
| [73] |
(a) Genêt, J.P.; Blart, E.; Savignac, M.; Lemeune, S.; Paris, J.-M. Tetrahedron Lett. 1993, 34,4189.
|
|
(b) Lemaire-Audoire, S.; Savignac, M.; Blart, E.; Pourcelot, G.; Genêt, J.P.; Bernard, J.-M. Tetrahedron Lett. 1994, 35,8783.
|
|
|
(c) Genêt, J.P.; Blart, E.; Savignac, M.; Lemeune, S.; Lemaire-Audoire, S.; Paris, J.-M.; Bernard, J.-M. Tetrahedron 1994, 50,497.
|
|
|
(d) Lemaire-Audoire, S.; Savignac, M.; Pourcelot, G.; Genet, J.-P.; Bernard, J.-M. J. Mol. Catal. A: Chem. 1997, 116,247.
|
|
| [74] |
Seki, M.; Kondo, K.; Kuroda, T.; Yamanaka, T.; Iwasaki, T. Synlett 1995,609.
|
| [75] |
Murakami, H.; Minami, T.; Ozawa, F. J. Org. Chem. 2004, 69,4482.
pmid: 15202905 |
| [76] |
Mora, G.; Piechaczyk, O.; Le Goff, X.F.; Le Floch, P. Organometallics 2008, 27,2565.
|
| [77] |
Mao, Y.; Liu, Y.; Hu, Y.; Wang, L.; Zhang, S.; Wang, W. ACS Catal. 2018, 8,3016.
|
| [78] |
Enugala, R.; Carvalho, L.C. R.; Marques, M.M. B. Synlett 2010,2711.
|
| [79] |
Martínez-Calvo, M.; Couceiro, J.R.; Destito, P.; Rodríguez, J.; Mosquera, J.; Mascareñas, J.L. ACS Catal. 2018,8.
|
| [80] |
Garner, A.L.; Song, F.; Koide, K. J. Am. Chem. Soc. 2009, 131,5163.
|
| [81] |
Nieberding, M.; Tracey, M.P.; Koide, K. ACS Sens. 2017, 2,1737.
pmid: 29058887 |
| [82] |
Jbara, M.; Eid, E.; Brik, A. Org. Biomol. Chem. 2018, 16,4061.
pmid: 29766191 |
| [83] |
Alcaide, B.; Almendros, P.; Alonso, J.M. Chem.-Eur. J. 2003, 9,5793.
pmid: 14673850 |
| [84] |
Alcaide, B.; Almendros, P.; Alonso, J.M. Chem.-Eur. J. 2006, 12,2874.
pmid: 16419144 |
| [85] |
Tanaka, S.; Saburi, H.; Ishibashi, Y.; Kitamura, M. Org. Lett. 2004, 6,1873.
pmid: 15151436 |
| [86] |
Tanaka, S.; Saburi, H.; Kitamura, M. Adv. Synth. Catal. 2006, 348,375.
|
| [87] |
Tanaka, S.; Saburi, H.; Murase, T.; Yoshimura, M.; Kitamura, M. J. Org. Chem. 2006, 71,4682.
|
| [88] |
Cadierno, V.; Garcia-Garrido, S.E.; Gimeno, J.; Nebra, N. Chem. Commun. 2005,4086.
|
| [89] |
Kamijo, S.; Huo, Z.; Jin, T.; Kanazawa, C.; Yamamoto, Y. J. Org. Chem. 2005, 70,6389.
|
| [90] |
Kajihara, K.; Arisawa, M.; Shuto, S. J. Org. Chem. 2008, 73,9494.
pmid: 18980332 |
| [91] |
Sasmal, P.K.; Carregal-Romero, S.; Parak, W.J.; Meggers, E. Organometallics 2012, 31,5968.
|
| [92] |
Rodriguez, J.G.; Canoira, L. React. Kinet. Catal. Lett. 1989, 38,351.
|
| [93] |
Chouhan, M.; Kumar, K.; Sharma, R.; Grover, V.; Nair, V.A. Tetrahedron Lett. 2013, 54,4540.
|
| [94] |
Taniguchi, T.; Ogasawara, K. Angew. Chem., nt. Ed. 1998, 37,1136.
|
| [95] |
Taniguchi, T.; Ogasawara, K. Tetrahedron Lett. 1998, 39,4679.
|
| [96] |
Kim, S.; Jo, J.; Lee, D. Org. Lett. 2016, 18,4530.
doi: 10.1021/acs.orglett.6b02140 pmid: 27560493 |
| [97] |
Gaertner, D.; Konnerth, H.; von Wangelin, A.J. Catal. Sci. Technol. 2013, 3,2541.
|
| [98] |
Iqbal, J.; Srivastava, R.R. Tetrahedron 1991, 47,3155.
|
| [99] |
Giedyk, M.; Turkowska, J.; Lepak, S.; Mar-culewicz, M.; Proinsias, K.O.; Gryko, D. Org. Lett. 2017, 19,2670.
doi: 10.1021/acs.orglett.7b01012 |
| [100] |
Hemming, D.S.; Talbot, E.P.; Steel, P.G. Tetrahedron Lett. 2017, 58,17.
doi: 10.1016/j.tetlet.2016.11.084 |
| [101] |
Torii, S.; Tanaka, H.; Katoh, T.; Morisaki, K. Tetrahedron Lett. 1984, 25,3207.
doi: 10.1016/S0040-4039(01)91010-X |
| [102] |
Espanet, B.; Duñach, E.; Périchon, J. Tetrahedron Lett. 1992, 33,2485.
|
| [103] |
Olivero, S.; Duñach, E. J. Chem. Soc., hem. Commun. 1995,2497.
|
| [104] |
Yasuhara, A.; Kasano, A.; Sakamoto, T. J. Org. Chem. 1999, 64,4211.
|
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||