有机化学 ›› 2021, Vol. 41 ›› Issue (4): 1359-1395.DOI: 10.6023/cjoc202008027 上一篇 下一篇
综述与进展
收稿日期:2020-08-15
修回日期:2020-10-22
发布日期:2020-11-19
通讯作者:
何炜
基金资助:Received:2020-08-15
Revised:2020-10-22
Published:2020-11-19
Contact:
Wei He
About author:Supported by:文章分享

现代有机合成中, C—H功能化反应可以用于直接构建各种生物活性骨架以及增加目标分子结构的复杂性, 是对传统合成方法的有效补充, 其中惰性C(sp3)—H功能化长期以来是该领域的难点. 目前, 碘试剂已被确定为经济和生态上无害的过渡金属替代品, 在具有挑战性的C(sp3)—H功能化反应中已经取得了卓有成效的进展. 分子碘与高价芳基碘试剂、二甲基亚砜(DMSO)、过氧化物以及氧气等组合, 能够高效地催化不同反应类型的C(sp3)—H功能化反应, 在不同氧化体系下具有不同的反应特点和机制. 另外, 近年来, 过渡金属/分子碘协同催化以及含碘电化学催化等新型碘催化体系在C(sp3)—H功能化反应中也得到了前所未有的发展. 综述了2015年至今分子碘在不同氧化体系下催化的C(sp3)—H功能化反应, 希望能进一步了解分子碘的绿色催化体系, 为深入研究C(sp3)—H功能化反应提供帮助.
张露文, 何炜. 分子碘催化氧化C(sp3)—H功能化反应的研究进展[J]. 有机化学, 2021, 41(4): 1359-1395.
Luwen Zhang, Wei He. Research Progress in C(sp3)—H Functionalization Reaction via Molecular Iodine-Catalyzed Oxidation[J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1359-1395.
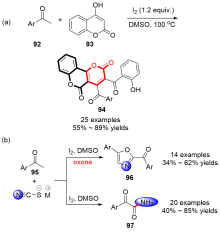
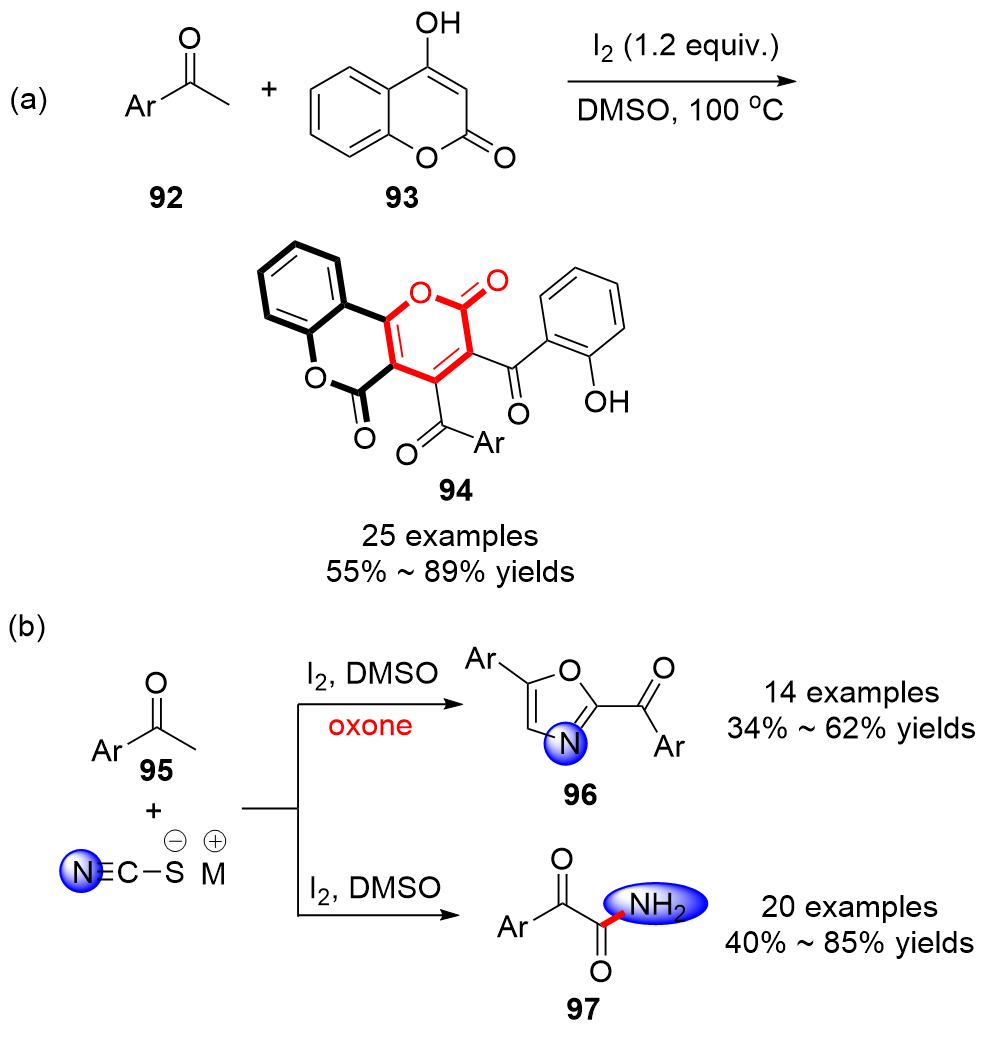

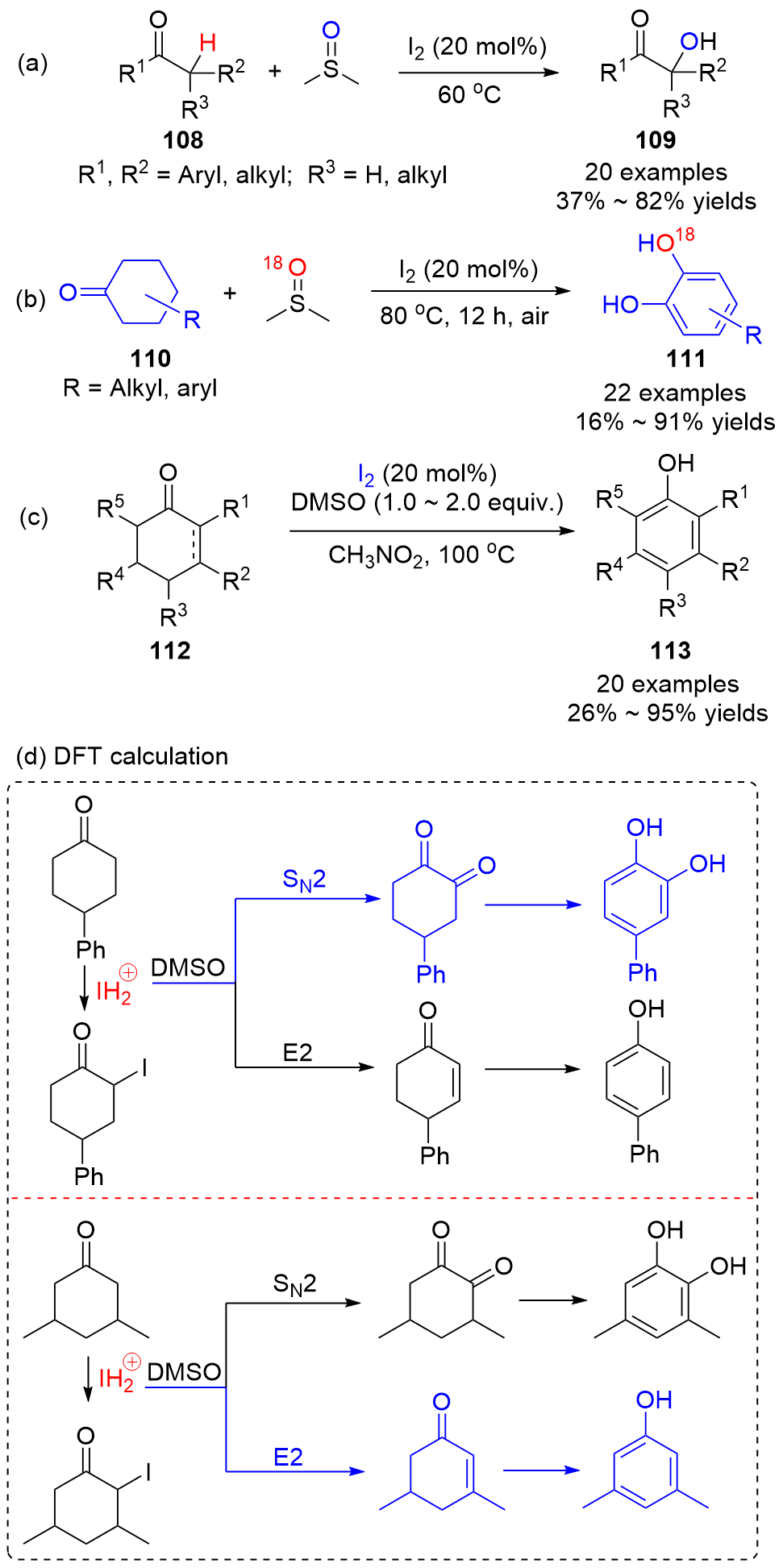

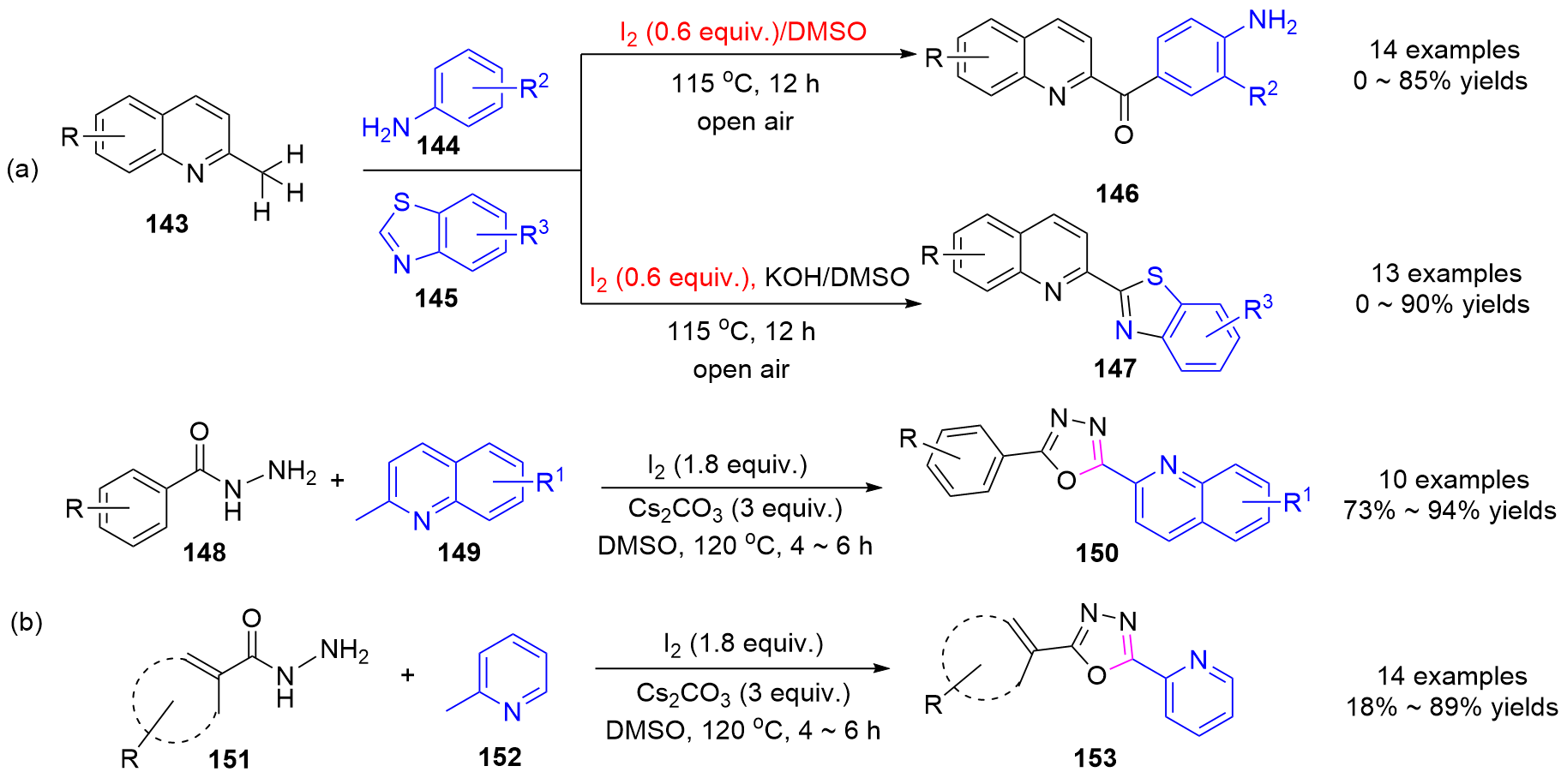






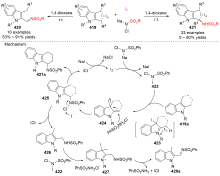
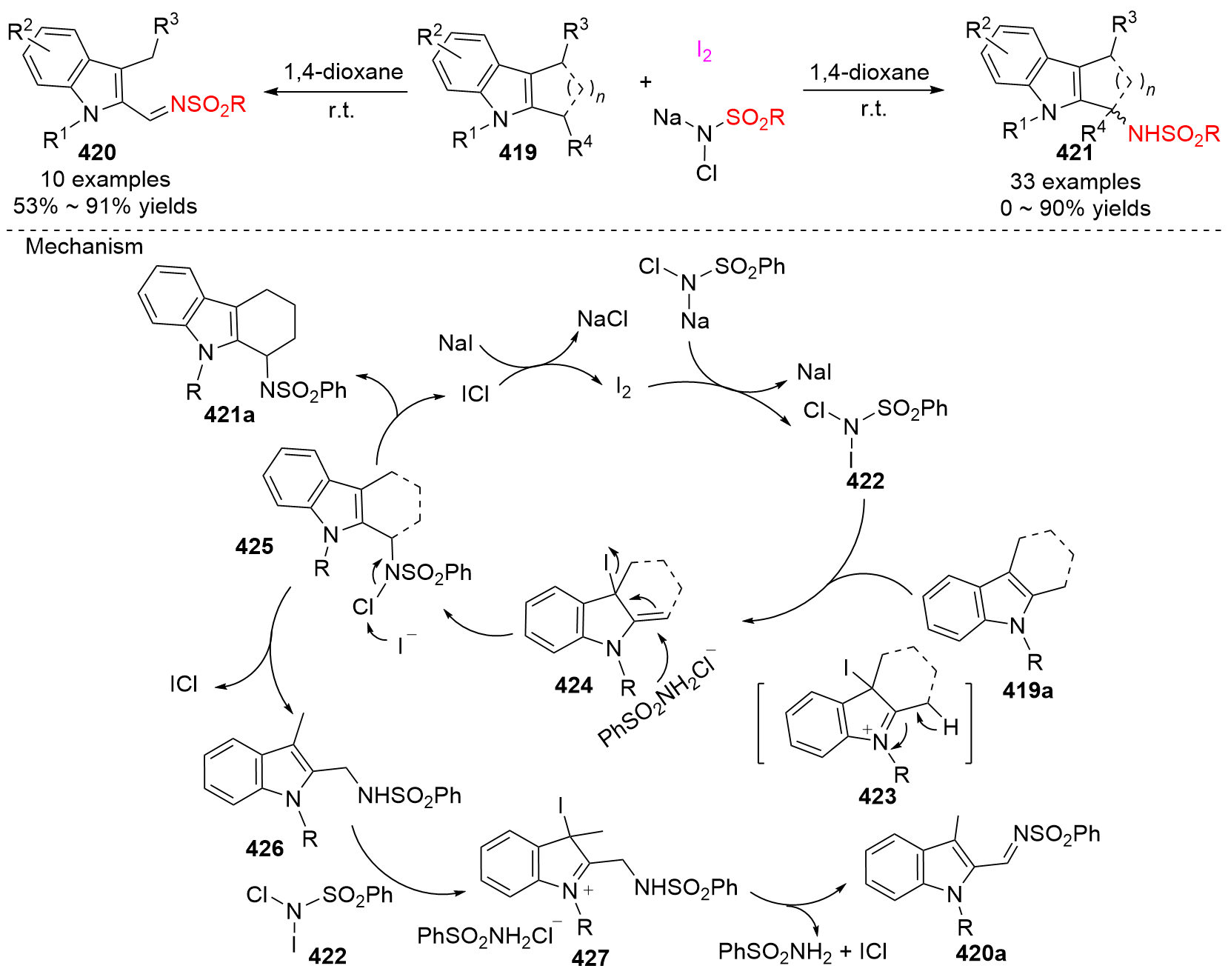
| [1] |
Togo, H.; Iida, S. Synlett 2006, 2006,2159.
|
| [2] |
Ren, Y.M.; Cai, C.; Yang, R.C. RSC Adv. 2013, 3,7182.
|
| [3] |
Chu, C.M.; Gao, S.J.; Sastry, M.N. V.; Yao, C.F. Tetrahedron Lett. 2005, 46,4971.
|
| [4] |
Yin, G.D.; Fan, L.; Ren, T.B.; Zheng, C.Y.; Tao, Q.; Wu, A.X.; She, N.F. Org. Biomol. Chem. 2012, 10,8877.
|
| [5] |
Wang, S.Y.; Ji, S.J.; Loh, T.P. Synlett 2003, 2003,2377.
|
| [6] |
Parvatkar, P.T.; Manetsch, R.; Banik, B.K. Chem.-Asian J. 2019, 14,6.
|
| [7] |
Mphahlele, M.J. Molecules 2009, 14,5308.
pmid: 20032894 |
| [8] |
Gaikwad, S.; Kamble, D.; Lokhande, P. Tetrahedron Lett. 2018, 59,2387.
|
| [9] |
Humne, V.; Dangat, Y.; Vanka, K.; Lokhande, P. Org. Biomol. Chem. 2014, 12,4832.
pmid: 24938996 |
| [10] |
Stateman, L.M.; Nakafuku, K.M.; Nagib, D.A. Synthesis 2018, 50,1569.
pmid: 29755145 |
| [11] |
Zhao, Y.Y.; Wang, E.B.; Wang, Y.L. Chin. J. Org. Chem. 2017, 37,866. (in Chinese)
|
|
( 赵玉英, 王二兵, 王颖莉, 有机化学, 2017, 37,866.)
|
|
| [12] |
Shi, Z.J.; Wang, L.H.; Cui, X.L. Chin. J. Org. Chem. 2019, 39,1596. (in Chinese)
|
|
( 施兆江, 王连会, 崔秀灵, 有机化学, 2019, 39,1596.)
|
|
| [13] |
Xu, X.M.; Chen, D.M.; Wang, Z.L. Chin. J. Org. Chem. 2019, 39,3338. (in Chinese)
|
|
( 徐鑫明, 陈德茂, 王祖利, 有机化学, 2019, 39,3338.)
|
|
| [14] |
Du, X.P.; Zhang, X.; Hou, F.; Liu, X.J.; Wang, X.C.; Quan, Z.J. Chin. J. Org. Chem. 2020, 40,1337. (in Chinese)
|
|
( 杜兴鹏, 张玺, 候飞, 刘小军, 王喜存, 权正军, 有机化学, 2020, 40,1337.)
|
|
| [15] |
Yang, L.F.; Hui, R.J.; Shan, H.Y.; Gong, K. Chin. J. Org. Chem. 2017, 37,3242. (in Chinese)
|
|
( 杨丽烽, 惠人杰, 单恒悦, 巩凯, 有机化学, 2017, 37,3242.)
|
|
| [16] |
Blanksby, S.J.; Ellison, G.B. Acc. Chem. Res. 2003, 36,255.
pmid: 12693923 |
| [17] |
Simões, J.A. M.; Beauchamp, J.L. Chem. Rev. 1990, 90,629.
|
| [18] |
Siegbahn, P.E. M. J. Phys. Chem. 1995, 99,12723.
|
| [19] |
Yeung, C.S.; Dong, V.M. Chem. Rev. 2011, 111,1215.
pmid: 21391561 |
| [20] |
Beccalli, E.M.; Broggini, G.; Martinelli, M.; Sottocornola, S. Chem. Rev. 2007, 107,5318.
doi: 10.1021/cr068006f pmid: 17973536 |
| [21] |
Hosseinian, A.; Ahmadi, S.; Nasab, F.A. H.; Mohammadi, R.; Vessally, E. Top. Curr. Chem. 2018, 376,1.
|
| [22] |
Li, C.J.; Li, Z.P. Pure Appl. Chem. 2006, 78,935.
|
| [23] |
Borpatra, P.J.; Deka, B.; Deb, M.L.; Baruah, P.K. Org. Chem. Front. 2019, 6,3445.
|
| [24] |
Chu, J.C. K.; Rovis, T. Angew. Chem., Int. Ed. 2017, 56,2.
|
| [25] |
Meng, G.Y.; Guo, T.J.; Ma, T.C.; Zhang, J.; Shen, Y.C.; Sharpless, K.B.; Dong, J.J. Nature 2019, 574,86.
|
| [26] |
Wu, X.; Gao, Q.H.; Geng, X.; Zhang, J.J.; Wu, Y.D.; Wu, A.X. Org. Lett. 2016, 18,2507.
pmid: 27181791 |
| [27] |
Stateman, L.M.; Wappes, E.A.; Nakafuku, K.M.; Edwards, K.M.; Nagib, D.A. Chem. Sci. 2019, 10,2693.
doi: 10.1039/c8sc05685d pmid: 30996986 |
| [28] |
Long, J.G.; Cao, X.; Zhu, L.Z.; Qiu, R.H.; Au, C.T.; Yin, S.F.; Iwasaki, T.; Kambe, N. Org. Lett. 2017, 19,2793.
pmid: 28508641 |
| [29] |
Siddaraju, Y.; Prabhu, K.R. Org. Lett. 2016, 18,6090.
|
| [30] |
Liang, Y.F.; Li, X.Y.; Wang, X.Y.; Zou, M.C.; Tang, C.H.; Liang, Y.J.; Song, S.; Jiao, N. J. Am. Chem. Soc. 2016, 138,12271.
doi: 10.1021/jacs.6b07269 |
| [31] |
Reddy, P.N.; Reddy, B.V. S.; Padmaja, P. Curr. Org. Synth. 2018, 15,815.
|
| [32] |
Monga, A.; Bagchi, S.; Sharma, A. New J. Chem. 2018, 42,1551.
|
| [33] |
Tashrifi, Z.; Khanaposhtani, M.M.; Larijani, B.; Mahdavi, M. Adv. Synth. Catal. 2020, 362,65.
|
| [34] |
Sudo, Y.; Yamaguchi, E.; Itoh, A. Org. Lett. 2017, 19,1610.
doi: 10.1021/acs.orglett.7b00428 pmid: 28322569 |
| [35] |
Rong, H.J.; Yao, J.J.; Li, J.K.; Qu, J. J. Org. Chem. 2017, 82,5557.
doi: 10.1021/acs.joc.7b00361 pmid: 28497692 |
| [36] |
Huang, H.Y.; Wu, H.R.; Wei, F.; Wang, D.; Liu, L. Org. Lett. 2015, 17,3702.
pmid: 26214240 |
| [37] |
Qian, P.; Zha, Z.G.; Wang, Z.Y. ChemElectroChem 2020, 12,2527.
|
| [38] |
Yang, L.; Xu, G.H.; Ma, J.J.; Yang, Q.; Feng, A.; Cui, J.G. Chin. J. Org. Chem. 2020, 40,28. (in Chinese)
|
|
( 杨柳, 许国贺, 马晶军, 杨倩, 冯安, 崔景港, 有机化学, 2020, 40,28.)
|
|
| [39] |
Hofmann, A.W. Chem. Ber. 1883, 16,558.
|
| [40] |
Armas, P.D.; Carrau, R.; Concepción, J.I.; Francisco, C.G.; Hernández, R.; Suárez, E. Tetrahedron Lett. 1985, 26,2493.
|
| [41] |
Dorta, R.L.; Francisco, C.G.; Suárez, E. J. Chem. Soc., hem. Commun. 1989,1168.
|
| [42] |
Francisco, C.G.; Herrera, A.J.; Kennedy, A.R.; Melián, D.; Suárez, E. Angew. Chem., Int. Ed. 2002, 41,856.
|
| [43] |
Paz, N.R.; Rodríguez-Sosa, D.; Valdés, H.; Marticorena, R.; Melián, D.; Copano, M.B.; González, C.C.; Herrera, A.J. Org. Lett. 2015, 17,2370.
doi: 10.1021/acs.orglett.5b00866 pmid: 25932748 |
| [44] |
Martínez, C.; Muñiz, K. Angew. Chem., Int. Ed. 2015, 54,8287.
|
| [45] |
Castillo, E.D.; Muñiz, K. Org. Lett. 2019, 21,705.
doi: 10.1021/acs.orglett.8b03909 pmid: 30672295 |
| [46] |
Barluenga, J.; González, J.M.; Campos, P.J.; Asensio, G. Angew. Chem., Int. Ed. 1985, 24,319.
|
| [47] |
Zhdankin, V.V.; Koposov, A.Y.; Yashin, N.V. Tetrahedron Lett. 2002, 43,5735.
|
| [48] |
Carlsson, A.C. C.; Mehmeti, K.; Uhrbom, M.; Karim, A.; Bedin, M.; Puttreddy, R.; Kleinmaier, R.; Neverov, A.A.; Nekoueishahraki, B.; Gräfenstein, J.; Rissanen, K.; Erdélyi, M. J. Am. Chem. Soc. 2016, 138,9853.
|
| [49] |
Duhamel, T.; Martínez, M.D.; Sideri, I.K.; Muñiz, K. ACS Catal. 2019, 9,7741.
|
| [50] |
Bosnidou, A.E.; Duhamel, T.; Muñiz, K. Eur. J. Org. Chem. 2020, 2020,6361.
|
| [51] |
Bosnidou, A.E.; Muñiz, K. Angew. Chem., Int. Ed. 2019, 58,7485.
|
| [52] |
Zhang, D.; Wang, H.; Cheng, H.C.; Hernández, J.G.; Bolm, C. Adv. Synth. Catal. 2017, 359,4274.
|
| [53] |
Genovino, J.; Lütz, S.; Sames, D.; Touré, B.B. J. Am. Chem. Soc. 2013, 135,12346.
doi: 10.1021/ja405471h |
| [54] |
Ripper, J.A.; Tiekink, E.R. T.; Scammells, P.J. Bioorg. Med. Chem. Lett. 2001, 11,443. 4ea89b7f-29df-4105-8bdc-665e2f95ef6e
doi: 10.1016/S0960-894X(00)00690-9 pmid: 11229743 |
| [55] |
Lancefield, C.S.; Zhou, L.; Lébl, T.; Slawin, A.M. Z.; Westwood, N.J. Org. Lett. 2012, 14,6166.
doi: 10.1021/ol302859j pmid: 23214465 |
| [56] |
Shu, X.Z.; Xia, X.F.; Yang, Y.F.; Ji, K.G.; Liu, X.Y.; Liang, Y.M. J. Org. Chem. 2009, 74,7464.
pmid: 19731925 |
| [57] |
Zhu, Y.; Shao, L.D.; Deng, Z.T.; Bao, Y.; Shi, X.; Zhao, Q.S. J. Org. Chem. 2018, 83,10166.
doi: 10.1021/acs.joc.8b01424 pmid: 30032617 |
| [58] |
Kanyiva, K.S.; Tane, M.; Shibata, T. J. Org. Chem. 2019, 84,12773.
pmid: 31313588 |
| [59] |
Wappes, E.A.; Nakafuku, K.M.; Nagib, D.A. J. Am. Chem. Soc. 2017, 139,10204.
doi: 10.1021/jacs.7b05214 pmid: 28741940 |
| [60] |
Prusinowski, A.F.; Twumasi, R.K.; Wappes, E.A.; Nagib, D.A. J. Am. Chem. Soc. 2020, 142,5429.
pmid: 32141741 |
| [61] |
Zhao, P.; Wu, X.; Geng, X.; Wang, C.; Zhou, Y.; Wu, Y.D.; Wu, A.X. J. Org. Chem. 2019, 84,8322.
doi: 10.1021/acs.joc.9b01160 pmid: 31140280 |
| [62] |
Monga, A.; Bagchi, S.; Sharma, A. New J. Chem. 2018, 42,1551.
|
| [63] |
Gao, Q.H.; Liu, S.; Wu, X.; Zhang, J.J.; Wu, A.X. J. Org. Chem. 2015, 80,5984.
doi: 10.1021/acs.joc.5b00785 pmid: 25973528 |
| [64] |
Gao, Q.H.; Liu, S.; Wu, X.; Wu, A.X. Org. Lett. 2014, 16,4582.
pmid: 25119142 |
| [65] |
Wu, X.; Geng, X.; Zhao, P.; Zhang, J.J.; Wu, Y.D.; Wu, A.X. Chem. Commun. 2017, 53,3438.
|
| [66] |
Wu, X.; Geng, X.; Zhao, P.; Zhang, J.J.; Gong, X.X.; Wu, Y.D.; Wu, A.X. Org. Lett. 2017, 19,1550.
doi: 10.1021/acs.orglett.7b00361 pmid: 28301171 |
| [67] |
Wu, X.; Zhao, P.; Geng, X.; Zhang, J.J.; Gong, X.X.; Wu, Y.D.; Wu, A.X. Org. Lett. 2017, 19,3319.
pmid: 28590762 |
| [68] |
Wu, X.; Geng, X.; Zhao, P.; Wu, Y.D.; Wu, A.X. Org. Lett. 2017, 19,4584.
pmid: 28832165 |
| [69] |
Xue, W.J.; Gao, Q.H.; Wu, A.X. Tetrahedron Lett. 2015, 56,7115.
|
| [70] |
Liu, S.; Xi, H.L.; Zhang, J.J.; Wu, X.; Gao, Q.H.; Wu, A.X. Org. Biomol. Chem. 2015, 13,8807.
doi: 10.1039/c5ob01313e pmid: 26214059 |
| [71] |
Wu, X.; Gao, Q.H.; Geng, X.; Zhang, J.J.; Wu, Y.D.; Wu, A.X. Org. Lett. 2016, 18,2507.
pmid: 27181791 |
| [72] |
Qun, C.; Zhuang, S.Y.; Yang, M.; Peng, N.; Liu, Y.; Wu, A.X. Tetrahedron 2019, 75,130756.
|
| [73] |
Wang, C.; Geng, X.; Zhao, P.; Zhou, Y.; Wu, Y.D.; Wu, A.X. Org. Chem. Front. 2019, 6,2534.
|
| [74] |
Weng, W.Z.; Gao, Y.H.; Zhang, X.; Liu, Y.H.; Shen, Y.J.; Zhu, Y.P.; Sun, Y.Y.; Meng, Q.G.; Wu, A.X. Org. Biomol. Chem. 2019, 17,2087.
pmid: 30702121 |
| [75] |
Zhang, Z.Y.; Xie, C.X.; Tan, X.C.; Song, G.L.; Wen, L.L.; Gao, H.; Ma, C. Org. Chem. Front. 2015, 2,942.
|
| [76] |
Xie, C.X.; Zhang, Z.Y.; Yang, B.C.; Song, G.L.; Gao, H.; Wen, L.L.; Ma, C. Tetrahedron 2015, 71,1831.
|
| [77] |
Satish, G.; Polu, A.; Ramar, T.; Ilangovan, A. J. Org. Chem. 2015, 80,5167.
doi: 10.1021/acs.joc.5b00581 pmid: 25906247 |
| [78] |
Liang, Y.F.; Wu, K.; Song, S.; Li, X.Y.; Huang, X.Q.; Jiao, N. Org. Lett. 2015, 17,876.
doi: 10.1021/ol5037387 pmid: 25650782 |
| [79] |
Liang, Y.F.; Li, X.Y.; Wang, X.Y.; Zou, M.C.; Tang, C.H.; Liang, Y.J.; Song, S.; Jiao, N. J. Am. Chem. Soc. 2016, 138,12271.
pmid: 27564642 |
| [80] |
Liang, Y.F.; Song, S.; Ai, L.S.; Li, X.W.; Jiao, N. Green Chem. 2016, 18,6462.
|
| [81] |
Mupparapu, N.; Vishwakarma, R.A.; Ahmed, Q.N. Tetrahedron 2015, 71,3417.
|
| [82] |
Yang, L.; Shi, X.; Hu, B.Q.; Wang, L.X. Asian J. Org. Chem. 2016, 5,494.
|
| [83] |
Sun, P.F.; Yang, D.S.; Wei, W.; Sun, X.J.; Zhang, W.H.; Zhang, H.; Wang, Y.; Wang, H. Tetrahedron 2017, 73,2022.
|
| [84] |
Siddaraju, Y.; Prabhu, K.R. Org. Lett. 2016, 18,6090.
doi: 10.1021/acs.orglett.6b03084 pmid: 27934388 |
| [85] |
Siddaraju, Y.; Prabhu, K.R. Org. Biomol. Chem. 2017, 15,5191.
pmid: 28590497 |
| [86] |
Chen, S.H.; Li, Y.; Chen, J.Y.; Xu, X.H.; Su, L.B.; Tang, Z.; Au, C.T.; Qiu, R.H. Synlett 2016, 27,2339.
|
| [87] |
Xi, L.Y.; Zhang, R.Y.; Shi, L.; Chen, S.Y.; Yu, X.Q. Beilstein J. Org. Chem. 2016, 12,1072.
doi: 10.3762/bjoc.12.101 pmid: 27340493 |
| [88] |
Xiong, M.T.; Gao, Z.; Liang, X.; Cai, P.F.; Zhu, H.P.; Pan, Y.J. Chem. Commun. 2018, 54,9679.
|
| [89] |
Barak, D.S.; Dighe, S.U.; Avasthi, I.; Batra, S. J. Org. Chem. 2018, 83,3537.
doi: 10.1021/acs.joc.7b03149 pmid: 29486127 |
| [90] |
Rahim, A.; Shaik, S.P.; Baig, M.F.; Alarifi, A.; Kamal, A. Org. Biomol. Chem. 2018, 16,635.
|
| [91] |
Mani, G.S.; Donthiboina, K.; Shankaraiah, N.; Kamal, A. New J. Chem. 2019, 43,15999.
|
| [92] |
Salihila, J.; Silva, L.; Pulgar, H.P. D.; Molina, A.Q.; González-Coloma, A.; Olmeda, A.S.; Moral, J. F., Q.D.; Barrero, A.F. J. Org. Chem. 2019, 84,6886.
doi: 10.1021/acs.joc.9b00704 pmid: 31083906 |
| [93] |
Chen, H.; Liu, L.; Huang, T.Z.; Chen, J.; Chen, T.Q. Adv. Synth. Catal. 2020, 362,3332.
|
| [94] |
Liu, M.; Chen, T.Q.; Zhou, Y.B.; Yin, S.F. Catal. Sci. Technol. 2016, 6,5792.
|
| [95] |
Liu, M.; Chen, X.; Chen, T.Q.; Xu, Q.; Yin, S.F. Org. Biomol. Chem. 2017, 15,9845.
doi: 10.1039/c7ob02490h pmid: 29135010 |
| [96] |
Chen, H.; Liu, L.; Huang, T.Z.; Jing, C.; Chen, T.Q. Org. Biomol. Chem. 2020, 16,3332.
|
| [97] |
Cao, Y.; Liu, L.; Huang, T.; Chen, T.Q. New J. Chem. 2020, 44,8697.
|
| [98] |
Jayram, J.; Xulu, B.A.; Jeena, V. Tetrahedron 2019, 75,130617.
|
| [99] |
Shang, Z.H.; Sun, J.; Guo, J.S.; Sun, Y.Y.; Weng, W.Z.; Zhang, Z.X.; Li, Z.J.; Zhu, Y.P. Tetrahedron 2020, 76,130887.
|
| [100] |
Grigoropoulou, G.; Clark, J.H.; Elings, J.A. Green Chem. 2003, 5,1.
|
| [101] |
Faveri, G.D.; Ilyashenko, G.; Watkinson, M. Chem. Soc. Rev. 2011, 40,1722.
doi: 10.1039/c0cs00077a pmid: 21079863 |
| [102] |
Tang, S.; Liu, K.; Long, Y.; Qi, X.T.; Lan, Y.; Lei, A.W. Chem. Commun. 2015, 51,8769.
|
| [103] |
Tang, S.; Liu, K.; Long, Y.; Gao, X.L.; Gao, M.; Lei, A.W. Org. Lett. 2015, 17,2404.
pmid: 25945514 |
| [104] |
Cao, H.; Yuan, J.W.; Liu, C.; Hu, X.Q.; Lei, A.W. RSC Adv. 2015, 5,41493.
|
| [105] |
Samanta, S.; Donthiri, R.R.; Dindaa, M.; Adimurthy, S. RSC Adv. 2015, 5,66718.
|
| [106] |
Vadagaonkar, K.S.; Kalmode, H.P.; Murugan, K.; Chaskar, A.C. RSC Adv. 2015, 5,5580.
|
| [107] |
Vadagaonkar, K.S.; Kalmode, H.P.; Prakash, S.; Chaskar, A.C. Synlett 2015, 26,1677.
|
| [108] |
Verma, A.; Patel, S.; Meenakshi; Kumar, A.; Yadav, A.; Kumar, S.; Jana, S.; Sharma, S.; Prasad, C.D.; Kumar, S. Chem. Commun. 2015, 51,1371.
|
| [109] |
Luo, W.K.; Shi, X.; Zhou, W.; Yang, L. Org. Lett. 2016, 18,2036.
pmid: 27123751 |
| [110] |
Shi, X.; Zhang, F.; Luo, W.K.; Yang, L. Synlett 2017, 28,494.
|
| [111] |
Luo, W.K.; Xu, C.L.; Yang, L. Tetrahedron Lett. 2019, 60,151328.
|
| [112] |
Shantharjun, B.; Rajeswari, R.; Vani, D.; Unnava, R.; Sridhar, B.; Reddy, K.R. Asian J. Org. Chem. 2019, 8,2162.
|
| [113] |
Zhu, D.; Luo, W.K.; Yang, L.; Ma, D.Y. Org. Biomol. Chem. 2017, 15,7112.
|
| [114] |
Chen, Z.K.; Li, H.L.; Dong, W.P.; Miao, M.Z.; Ren, H.J. Org. Lett. 2016, 18,1334.
pmid: 26914527 |
| [115] |
Wei, W.; Cui, H.H.; Yang, D.S.; Liu, X.X.; He, C.L.; Dai, S.C.; Wang, H. Org. Chem. Front. 2017, 4,26.
|
| [116] |
Chen, K.L.; Gao, B.; Shang, Y.G.; Du, J.Y.; Gu, Q.L.; Wang, J.X. Org. Biomol. Chem. 2017, 15,8770.
doi: 10.1039/c7ob02038d pmid: 29019500 |
| [117] |
Sun, K.; Lv, Y.H.; Chen, Y.H.; Zhou, T.T.; Xing, Y.Y.; Wang, X. Org. Biomol. Chem. 2017, 15,4464.
doi: 10.1039/c7ob00958e pmid: 28489107 |
| [118] |
Deb, M.L.; Borpatra, P.J.; Saikia, P.J.; Baruah, P.K. Synlett 2017, 28,461.
|
| [119] |
Long, J.G.; Cao, X.; Zhu, L.Z.; Qiu, R.H.; Au, C.T.; Yin, S.F.; Iwasaki, T.; Kambe, N. Org. Lett. 2017, 19,2793.
doi: 10.1021/acs.orglett.7b00846 pmid: 28508641 |
| [120] |
Yaragorla, S.; Babu, V.P. Tetrahedron Lett. 2017, 58,1879.
|
| [121] |
Wang, J.; Zhang, F.D.; Tang, D.; Wu, P.; Zhang, X.G.; Chen, B.H. RSC Adv. 2017, 7,24594.
|
| [122] |
Wei, W.T.; Zhu, W.M.; Bao, W.H.; Chen, W.T.; Huang, Y.L.; Gao, L.H.; Xu, X.D.; Wang, Y.N.; Chen, G.P. ACS Sustainable Chem. Eng. 2018, 6,5615.
|
| [123] |
Chen, C.; Liu, W.B.; Zhou, P.; Liu, H.L. RSC Adv. 2017, 7,20394.
|
| [124] |
Duhamel, T.; Stein, C.J.; Martínez, C.; Reiher, M.; Muñiz, K. ACS Catal. 2018, 8,3918.
|
| [125] |
Becker, P.; Duhamel, T.; Stein, C.J.; Reiher, M.; Muñiz, K. Angew. Chem., Int. Ed. 2017, 56,8004.
|
| [126] |
Duhamel, T.; Muñiz, K. Chem. Commun. 2019, 55,933.
|
| [127] |
Wang, H.X.; Wei, T.Q.; Xu, P.; Wang, S.Y.; Ji, S.J. J. Org. Chem. 2018, 83,13491.
doi: 10.1021/acs.joc.8b02395 pmid: 30339009 |
| [128] |
Liu, L.; Tan, C.; Fan, R.; Wang, Z.H.; Du, H.G.; Xu, K.; Tan, J.J. Org. Biomol. Chem. 2019, 17,252.
pmid: 30564820 |
| [129] |
Valeur, E.; Bradley, M. Chem. Soc. Rev. 2009, 38,606.
pmid: 19169468 |
| [130] |
El-Faham, A.; Albericio, F. Chem. Rev. 2011, 111,6557.
pmid: 21866984 |
| [131] |
Li, J.; Lear, M.J.; Kawamoto, Y.; Umemiya, S.; Wong, A.R.; Kwon, E.; Sato, I.; Hayashi, Y. Angew. Chem., Int. Ed. 2015, 127,13178.
|
| [132] |
Huang, H.Y.; Wu, H.R.; Wei, F.; Wang, D.; Liu, L. Org. Lett. 2015, 17,3702.
doi: 10.1021/acs.orglett.5b01662 pmid: 26214240 |
| [133] |
Nguyen, T.B.; Ermolenko, L.; Retailleau, P.; Al-Mourabit, A. Org. Lett. 2016, 18,2177.
pmid: 27088653 |
| [134] |
Sudo, Y.; Yamaguchi, E.; Itoh, A. Org. Lett. 2017, 19,1610.
pmid: 28322569 |
| [135] |
Yang, J.Y.; Xie, D.T.; Zhou, H.Y.; Chen, S.W.; Huo, C.D.; Li, Z. Org. Chem. Front. 2018, 5,1325.
|
| [136] |
Tuo, X.L.; Chen, S.P.; Jiang, P.Y.; Ni, P.H.; Wang, X.D.; Deng, G.J. RSC Adv. 2020, 10,8348.
|
| [137] |
Lv, Y.H.; Sun, K.; Wang, T.T.; Li, G.; Pu, W.Y.; Chai, N.N.; Shen, H.H.; Wu, Y.T. RSC Adv. 2015, 7,72142.
|
| [138] |
Lv, Y.H.; Sun, K.; Wang, T.T.; Wu, Y.T.; Li, G.; Pu, W.Y.; Mao, S.K. Asian J. Org. Chem. 2016, 5,325.
|
| [139] |
Lv, Y.H.; Pu, W.Y.; Niu, J.J.; Wang, Q.Q.; Chen, Q. Tetrahedron Lett. 2018, 59,1497.
|
| [140] |
Zhou, Y.; Chen, J.W.; Elsayed, A.A.; Zhang, Z.G.; Bao, Z.B.; Yang, Q.W.; Yang, Y.W.; Ren, Q.L. Catal. Lett. 2019, 149,574.
|
| [141] |
Sridhar, A.; Selvaraj, M. ChemistrySelect 2019, 4,1890.
|
| [142] |
Zhang, J.; Zhao, X.; Liu, P.; Sun, P.P. J. Org. Chem. 2019, 84,12596.
doi: 10.1021/acs.joc.9b02145 pmid: 31502839 |
| [143] |
Tan, C.; Liu, Y.G.; Liu, X.Y.; Jia, H.X.; Xu, K.; Huang, S.H.; Wang, W.J.; Tan, J.J. Org. Chem. Front. 2020, 7,780.
|
| [144] |
Castanedo, G.; Liu, Y.Z.; Crawford, J.J.; Braun, M.G. J. Org. Chem. 2016, 81,8617.
doi: 10.1021/acs.joc.6b01517 pmid: 27529722 |
| [145] |
Wang, P.; Gao, X.L.; Huang, P.F.; Lei, A.W. ChemCatChem 2020, 1,27.
|
| [146] |
Hu, X.; Nie, L.; Zhang, G.T.; Lei, A.W. Angew. Chem., Int. Ed. 2020, 59,15238.
|
| [147] |
Tang, S.; Wang, S.Y.; Liu, Y.C.; Cong, H.J.; Lei, A.W. Angew. Chem., Int. Ed. 2018, 57,4737.
|
| [148] |
Liu, K.; Song, C.L.; Wu, J.R.; Deng, Y.Q.; Tang, S.; Lei, A.W. Green Chem. 2019, 21,765.
|
| [149] |
Liang, S.; Zeng, C.C.; Tian, H.Y.; Sun, B.G.; Luo, X.G.; Ren, F.Z. J. Org. Chem. 2016, 81,11565.
pmid: 27934459 |
| [150] |
Liang, S.; Zeng, C.C.; Tian, H.Y.; Sun, B.G.; Luo, X.G.; Ren, F.Z. Adv. Synth. Catal. 2018, 360,1444.
|
| [151] |
Qian, P.; Yan, Z.; Zhou, Z.H.; Hu, K.F.; Wang, J.W.; Li, Z.B.; Zha, Z.G.; Wang, Z.Y. Org. Lett. 2018, 20,6359.
doi: 10.1021/acs.orglett.8b02578 pmid: 30280901 |
| [152] |
Qian, P.; Zhou, Z.H.; Hu, K.F.; Wang, J.W.; Li, Z.B.; Zha, Z.G.; Wang, Z.Y. Org. Lett. 2019, 21,6403.
pmid: 31361492 |
| [153] |
Wang, J.W.; Qian, P.; Hu, K.F.; Zha, Z.G.; Wang, Z.Y. ChemElectroChem 2019, 16,4292.
|
| [154] |
Rafiee, M.; Wang, F.; Hruszkewycz, D.P.; Stahl, S.S. J. Am. Chem. Soc. 2017, 140,22.
doi: 10.1021/jacs.7b09744 pmid: 29220181 |
| [155] |
Wang, H.Q.; Zhang, J.J.; Tan, J.J.; Xin, L.L.; Li, Y.P.; Zhang, S.; Xu, K. Org. Lett. 2018, 20,2505.
pmid: 29664646 |
| [156] |
Mohan, D.C.; Ravi, C.; Pappula, V.; Adimurthy, S. J. Org. Chem. 2015, 80,6846.
doi: 10.1021/acs.joc.5b00477 pmid: 26044904 |
| [157] |
Zhao, Z.G.; Wang, T.; Yuan, L.; Ji, X.W.; Zhao, J.F. RSC Adv. 2015, 5,75386.
|
| [158] |
Gao, Q.H.; He, S.; Wu, X.; Zhang, J.J.; Bai, S.P.; Wu, Y.D.; Wu, A.X. Org. Chem. Front. 2018, 5,765.
|
| [159] |
Iwahama, T.; Syojyo, K.; Sakaguchi, S.; Ishii, Y. Org. Process Res. Dev. 1998, 2,255.
|
| [160] |
Wu, X.; Zhao, P.; Geng, X.; Wang, C.; Wu, Y.D.; Wu, A.X. Org. Lett. 2018, 20,688.
pmid: 29327934 |
| [161] |
Geng, X.; Wang, C.; Huang, C.; Bao, Y.; Zhao, P.; Zhou, Y.; Wu, Y.D.; Feng, L.L.; Wu, A.X. Org. Lett. 2020, 22,140.
doi: 10.1021/acs.orglett.9b04060 pmid: 31858804 |
| [162] |
Wu, X.; Zhang, J.J.; Liu, S.; Gao, Q.H.; Wu, A.X. Adv. Synth. Catal. 2016, 358,218.
|
| [163] |
Yi, X.L.; Xi, C.J. Tetrahedron 2017, 73,1311.
|
| [164] |
Ujwaldev, S.M.; Rohit, K.R.; Harry, N.A.; Anilkumar, G. Tetrahedron Lett. 2019, 13,150950.
|
| [165] |
Sangeetha, S.; Sekar, G. Org. Lett. 2018, 21,75.
pmid: 30511870 |
| [166] |
Sangeetha, S.; Sekar, G. Chem Commun. 2020, 56,10906.
|
| [167] |
Chen, L.S.; Zhang, L.B.; Tian, Y.; Li, J.H.; Liu, Y.Q. Eur. J. Org. Chem. 2020, 2020,5523.
|
| [168] |
Zhang, H.W.; Muñiz, K. ACS Catal. 2017, 7,4122.
|
| [169] |
Yavari, I.; Hosseinpour, R.; Skoulika, S. Synlett 2015, 26,380.
|
| [170] |
Usami, K.; Nagasawa, Y.; Yamaguchi, E.; Tada, N.; Itoh, A. Org. Lett. 2016, 18,8.
pmid: 26654114 |
| [171] |
Reddy, N.N. K.; Mohan, D.C.; Adimurthy, S. Tetrahedron Lett. 2016, 57,1074.
|
| [172] |
Pang, X.B.; Xiang, L.K.; Ma, J.X.; Yang, X.D.; Yan, R.L. RSC Adv. 2016, 6,111713.
|
| [173] |
Rong, H.J.; Yao, J.J.; Li, J.K.; Qu, J. J. Org. Chem. 2017, 82,5557.
pmid: 28497692 |
| [174] |
Liu, R.H.; Wang, Y.K.; Weng, Y.M.; Yao, C.P.; Zhang, Y.; Zhu, G.Z.; He, X.A.; Xu, K.P.; Tan, G.S. Synlett 2017, 28,1083.
|
| [175] |
Liu, X.Z.; Zhou, Y.X.; Yang, Z.Q.; Li, Q.; Zhao, L.; Liu, P.J. J. Org. Chem. 2018, 83,4665.
|
| [176] |
Zhang, J.; Zheng, T.T.; Zhang, J.D. Eur. J. Org. Chem. 2020, 2020,860.
|
| [1] | 黄净, 杨毅华, 张占辉, 刘守信. 酰胺键的绿色高效构建方法与技术进展[J]. 有机化学, 2024, 44(2): 409-420. |
| [2] | 徐利军, 李宗军, 韩福社, 高翔. N,N-二甲基甲酰胺促进的富勒烯稠合噁唑啉衍生物的合成[J]. 有机化学, 2024, 44(1): 242-250. |
| [3] | 岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博. 无支持电解质条件下连续流电化学合成三氟甲基化氧化吲哚[J]. 有机化学, 2023, 43(9): 3239-3245. |
| [4] | 蒋宜欣, 唐伯孝, 毛海波, 陈雪霞, 俞洋杰, 全翠英, 徐昭阳, 石金慧, 刘益林. 水-聚乙二醇(PEG-200)中烯烃与碘代芳烃绿色可循环无负载偶联反应的研究[J]. 有机化学, 2023, 43(9): 3210-3215. |
| [5] | 钟赟哲, 陈颖, 俞磊, 周宏伟. 电化学介导羧酸与醇的酯化反应[J]. 有机化学, 2023, 43(8): 2855-2863. |
| [6] | 张周, 郭钰, 羊静, 吴丹, 王佳昕, 洪欣玥, 蔡佩君, 荣良策. 电化学促进咪唑并[1,2-a]吡啶与二氯(溴)乙烷及碘仿的卤化反应[J]. 有机化学, 2023, 43(6): 2104-2109. |
| [7] | 卢凯, 屈浩琦, 陈樨, 秋慧, 郑晶, 马猛涛. 无催化剂、无溶剂条件下炔烃和烯烃与儿茶酚硼烷的硼氢化反应[J]. 有机化学, 2023, 43(6): 2197-2205. |
| [8] | 张俊颖, 赵晓静, 李干鹏, 何永辉. 室温下电化学合成保护型有机硼酸RB(dan)[J]. 有机化学, 2023, 43(5): 1815-1823. |
| [9] | 杜琳琳, 张华. 芳烃与烷烃化合物参与的光化学与电化学硼化反应[J]. 有机化学, 2023, 43(5): 1726-1741. |
| [10] | 莫百川, 陈春霞, 彭进松. 木质素及其衍生物负载金属催化剂在有机合成中的应用研究进展[J]. 有机化学, 2023, 43(4): 1215-1240. |
| [11] | 潘永周, 蒙秀金, 王迎春, 何慕雪. 电化学固定CO2构建羧酸衍生物的研究进展[J]. 有机化学, 2023, 43(4): 1416-1434. |
| [12] | 窦谦, 汪太民, 房丽晶, 翟宏斌, 程斌. 光诱导铁催化在有机合成中的应用研究进展[J]. 有机化学, 2023, 43(4): 1386-1415. |
| [13] | 孙丽, 宋国欣, 韩家乐, 李继玉, 赵月, 杨璐华, 张峰, 赵坤, 毛比明. Morita-Baylis-Hillman加合物和N-羟基邻苯二甲酰亚胺的电化学烯丙基烷基化形成C(sp3)—C(sp3)键[J]. 有机化学, 2023, 43(4): 1574-1583. |
| [14] | 黄嘉为, 李潇漫, 徐亮, 韦玉. α-酮酸与硫酚的电化学脱羧偶联: 一种合成硫代酸酯的新方法[J]. 有机化学, 2023, 43(2): 756-762. |
| [15] | 李奇阳, 张海燕, 刘文博. 无过渡金属参与的碳硅键构筑方法研究进展[J]. 有机化学, 2023, 43(10): 3470-3490. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
