有机化学 ›› 2022, Vol. 42 ›› Issue (7): 2098-2105.DOI: 10.6023/cjoc202203020 上一篇 下一篇
所属专题: 有机氟化学虚拟合辑
研究论文
曹成瑶a, 牛亚茹b, 蒋昀辰a, 曲红梅b, 陈超a,c,*( )
)
收稿日期:2022-03-10
修回日期:2022-04-08
发布日期:2022-08-09
通讯作者:
陈超
基金资助:
Chengyao Kimmy Caoa, Yaru Niub, Yunchen Jianga, Hongmei Qub, Chao Chena,c( )
)
Received:2022-03-10
Revised:2022-04-08
Published:2022-08-09
Contact:
Chao Chen
Supported by:文章分享
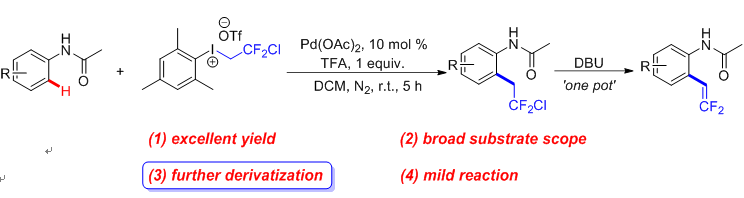
二氟乙基化合物在生物医药和材料领域有着广泛的应用, 合成二氟乙基的化合物有着重要意义, 在此报道了一种钯催化的乙酰苯胺邻位氯二氟乙基化的反应, 该反应利用氯二氟乙基高价碘试剂(CDFI)作为CH2CF2Cl的来源. 进一步研究发现, 有机碱1,8-二氮杂二环[5.4.0]十一碳-7-烯(DBU)加入到反应体系中, 可以通过消除反应制备乙酰苯胺邻位偕二氟乙烯基化产物.
曹成瑶, 牛亚茹, 蒋昀辰, 曲红梅, 陈超. 钯催化的氯二氟乙基高价碘试剂对于乙酰苯胺C—H键的氯二氟乙基化反应研究[J]. 有机化学, 2022, 42(7): 2098-2105.
Chengyao Kimmy Cao, Yaru Niu, Yunchen Jiang, Hongmei Qu, Chao Chen. Pd-Catalyzed Chlorodifluoroethylation Reaction of C—H Bond of Acetanilides with Chlorodifluoroethyl Iodonium Reagents[J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2098-2105.
| Entry | Catalyst | Solvent | Acid | NMR yieldb/% |
|---|---|---|---|---|
| 1 | Pd(OAc)2 | DCM | — | 30 |
| 2 | Pd(OAc)2 | DCM | AcOH | 32 |
| 3 | Pd(OAc)2 | DCM | TsOH | 61 |
| 4 | Pd(OAc)2 | DCM | BF3•Et2O | 32 |
| 5 | — | DCM | TFA | n.d. |
| 6 | PdCl2 | DCM | TFA | 15 |
| 7 | Pd2(dba)3 | DCM | TFA | 63 |
| 8 | Cu(OTf)2 | DCM | TFA | n.d. |
| 9 | Pd(OAc)2 | DCM | TFA | 93 |
| 10 | Pd(OAc)2 | MeOH | TFA | n.d. |
| 11 | Pd(OAc)2 | THF | TFA | 16 |
| 12 | Pd(OAc)2 | Toluene | TFA | 71 |
| 13 | Pd(OAc)2 | DCM | TFA | 99 |
| Entry | Catalyst | Solvent | Acid | NMR yieldb/% |
|---|---|---|---|---|
| 1 | Pd(OAc)2 | DCM | — | 30 |
| 2 | Pd(OAc)2 | DCM | AcOH | 32 |
| 3 | Pd(OAc)2 | DCM | TsOH | 61 |
| 4 | Pd(OAc)2 | DCM | BF3•Et2O | 32 |
| 5 | — | DCM | TFA | n.d. |
| 6 | PdCl2 | DCM | TFA | 15 |
| 7 | Pd2(dba)3 | DCM | TFA | 63 |
| 8 | Cu(OTf)2 | DCM | TFA | n.d. |
| 9 | Pd(OAc)2 | DCM | TFA | 93 |
| 10 | Pd(OAc)2 | MeOH | TFA | n.d. |
| 11 | Pd(OAc)2 | THF | TFA | 16 |
| 12 | Pd(OAc)2 | Toluene | TFA | 71 |
| 13 | Pd(OAc)2 | DCM | TFA | 99 |
| [1] |
(a) Caron, S. Org. Process Res. Dev. 2020, 24, 470.
doi: 10.1021/acs.oprd.0c00030 |
|
(b) Berger, R.; Resnati, G.; Hulliger, J. Chem. Soc. Rev. 2011, 40, 3496.
doi: 10.1039/c0cs00221f |
|
| [2] |
(a) Magueur, G.; Bonnet-Delpon, D.; Bégué, J. P. J. Fluorine Chem. 2006, 127, 637.
doi: 10.1016/j.jfluchem.2005.12.013 |
|
(b) Meanwell, N. A. J. Med. Chem. 2011, 54, 2529.
doi: 10.1021/jm1013693 |
|
|
(c) Torre de La, B. G.; Albericio, F. Molecules 2020, 25, 745.
doi: 10.3390/molecules25030745 |
|
| [3] |
Magueur, G.; Bonnet-Delpon, D.; Bégué, J. P. J. Fluorine Chem. 2006, 127, 637.
doi: 10.1016/j.jfluchem.2005.12.013 |
| [4] |
Karst, N. A.; Islam, T. F.; Linhardt, R. J. Org. Lett. 2003, 5, 4839.
doi: 10.1021/ol035882w |
| [5] |
Wang, W.; Liu, G.; Jiao, N. CCS Chem. 2020, 2, 566.
doi: 10.31635/ccschem.020.202000172 |
| [6] |
(a) Deng, X. Y.; Lin, J. H.; Xiao, J. C. Org. Lett. 2016, 18, 4384.
doi: 10.1021/acs.orglett.6b02141 pmid: 24605155 |
|
(b) Feng, Z.; Min, Q. Q.; Zhang, X. Nat. Chem. 2017, 9, 918.
doi: 10.1038/nchem.2746 pmid: 24605155 |
|
|
(c) Feng, Z.; Min, Q. Q.; Zhang, X. Angew. Chem., Int. Ed. 2014, 53, 1669.
doi: 10.1002/anie.201309535 pmid: 24605155 |
|
|
(d) Feng, Z.; Xiao, Y.L.; Zhang, X. Acc. Chem. Res. 2018, 51, 2264.
doi: 10.1021/acs.accounts.8b00230 pmid: 24605155 |
|
|
(e) Li, L.; Wang, F.; Hu, J. Angew. Chem., Int. Ed. 2020, 52, 12390.
doi: 10.1002/anie.201306703 pmid: 24605155 |
|
|
(f) Salomon, P.; Zard, S. Z. Org. Lett. 2014, 16, 2926.
doi: 10.1021/ol501063a pmid: 24605155 |
|
|
(g) Wang, F.; Li, L.; Hu, J. Beilstein. J. Org. Chem. 2014, 10, 344.
doi: 10.3762/bjoc.10.32 pmid: 24605155 |
|
|
(h) Wang, F.; Prakash, G. K.; Olah, G. A. Angew. Chem., Int. Ed. 2011, 50, 7153.
doi: 10.1002/anie.201101691 pmid: 24605155 |
|
|
(i) Zhang, H.; Lan, Y.; Liu, G. S. ACS Catalysis 2018, 8, 2173.
doi: 10.1021/acscatal.7b03220 pmid: 24605155 |
|
|
(j) Zheng, J.; Hu., J.; Federsel, H. J. Chem. Commun. 2007, 5149.
pmid: 24605155 |
|
| [7] |
(a) Zhang, X.; Cao, S. Tetrahedron Lett. 2017, 58, 375.
doi: 10.1016/j.tetlet.2016.12.054 pmid: 22085400 |
|
(b) Chelucci, G. Chem. Rev. 2012, 112, 1344.
doi: 10.1021/cr200165q pmid: 22085400 |
|
|
(c) Prakash, G. K. S.; Hu, J. B.; Olah, G. A. J. Fluorine Chem. 2006, 127, 1361.
pmid: 22085400 |
|
|
(d) Nowak, I.; Robins, M. J. Org. Lett. 2005, 7, 721.
doi: 10.1021/ol047416s pmid: 22085400 |
|
|
(e) Zhao, Y.; Zhu, L.; Hu, J. Org. Lett. 2010, 12, 1444.
doi: 10.1021/ol100090r pmid: 22085400 |
|
| [8] |
(a) Khake, S. M.; Chatani, N. Chem 2020, 6, 1056.
doi: 10.1016/j.chempr.2020.04.005 |
|
(b) He, J.; Shao, Q.; Yu, J. Q. Chem. Rev. 2017, 117, 8754.
doi: 10.1021/acs.chemrev.6b00622 |
|
|
(c) Rej, S.; Ano, Y.; Chatani, N. Chem. Rev. 2020, 120, 1788.
doi: 10.1021/acs.chemrev.9b00495 |
|
|
(d) Zhang, Q.; Shi, B. F. Chin. J. Chem. 2019, 37, 647.
doi: 10.1002/cjoc.201900090 |
|
|
(e) Gandeepan, P.; Warratz, S.; Ackermann, L. Chem. Rev. 2019, 119, 2192.
doi: 10.1021/acs.chemrev.8b00507 |
|
| [9] |
(a) Liu, L. Y.; Qiao, J. X.; Yu, J. Q. J. Am. Chem. Soc. 2019, 141, 14870.
doi: 10.1021/jacs.9b07887 pmid: 20307066 |
|
(b) Font, M.; Quibell, J. M.; Larrosa, I. Chem. Commun. 2017, 53, 5584.
doi: 10.1039/C7CC01755C pmid: 20307066 |
|
|
(c) Nishikata, T.; Huang, S.; Lipshutz, B. H. J. Am. Chem. Soc. 2010, 132, 4978.
doi: 10.1021/ja910973a pmid: 20307066 |
|
|
(d) Nishikata, T.; Abela, A. R.; Lipshutz, B. H. Angew. Chem., Int. Ed. 2010, 49, 781.
doi: 10.1002/anie.200905967 pmid: 20307066 |
|
|
(e) Jiang, Z.; Xu, L.; Fan, Q. RSC Adv. 2020, 3, 1025.
doi: 10.1039/C2RA22901C pmid: 20307066 |
|
|
(f) Jiang, P.; Yu, R.; Wang, Q. Org. Lett. 2015, 17, 5918.
doi: 10.1021/acs.orglett.5b03153 pmid: 20307066 |
|
| [10] |
(a) Aihara, Y.; Chatani, N. J. Am. Chem. Soc. 2020, 135, 5308.
doi: 10.1021/ja401344e pmid: 20175511 |
|
(b) Song, W.; Lackner, S.; Ackermann, L. Angew. Chem., Int. Ed. 2014, 53, 2477.
doi: 10.1002/anie.201309584 pmid: 20175511 |
|
|
(c) Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2010, 132, 3965.
doi: 10.1021/ja910900p pmid: 20175511 |
|
|
(d) Zhang, S. Y.; Li, Q.; Chen, G. J. Am. Chem. Soc. 2020, 135, 2124.
doi: 10.1021/ja312277g pmid: 20175511 |
|
|
(e) Zhang, S. Y.; Li, Q.; Chen, G. J. Am. Chem. Soc. 2020, 135, 12135.
doi: 10.1021/ja406484v pmid: 20175511 |
|
|
(f) Zhang, H.; Chen, P.; Liu, G. Angew. Chem., Int. Ed. 2014, 53, 10174.
doi: 10.1002/anie.201403793 pmid: 20175511 |
|
|
(g) Zhang, H.; Chen, P.; Liu, G. Angew. Chem., Int. Ed. 2014, 126, 10338.
pmid: 20175511 |
|
|
(h) Fujiwara, Y.; Ishihara, Y.; Baran, P. S. Nature 2012, 492, 95.
doi: 10.1038/nature11680 pmid: 20175511 |
|
| [11] |
(a) Yusubov, M. S.; Zhdankin, V. V. Curr. Org. Synth. 2012, 9, 247.
doi: 10.2174/157017912799829021 pmid: 33134246 |
|
(b) Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328.
doi: 10.1021/acs.chemrev.5b00547 pmid: 33134246 |
|
|
(c) Han, Y. C. Zhang, C. Tetrahedron Lett. 2018, 59, 3052.
doi: 10.1016/j.tetlet.2018.06.059 pmid: 33134246 |
|
|
(d) Bal, A.; Maiti, S.; Mal, P. Chem. Asian J. 2020, 15, 624.
doi: 10.1002/asia.201901683 pmid: 33134246 |
|
|
(e) Takenaga, N.; Kumar, R.; Dohi, T. Front. Chem. 2020, 8, 599026.
doi: 10.3389/fchem.2020.599026 pmid: 33134246 |
|
|
(f) Shetgaonkar, S. E.; Singh, F. V. Front. Chem. 2020, 8, 705.
doi: 10.3389/fchem.2020.00705 pmid: 33134246 |
|
|
(g) Wang, Y.; Chen, C.; Peng, J. Angew. Chem., Int. Ed. 2013, 52, 5323.
doi: 10.1002/anie.201300586 pmid: 33134246 |
|
|
(h) Dong, D. Q.; Hao, S. H.; Chen, C. Org. Biomol. Chem. 2014, 12, 4278.
doi: 10.1039/c4ob00318g pmid: 33134246 |
|
|
(i) Chen, J.; Chen, C; Qu, H. Chem. Commun. 2015, 51, 1356.
doi: 10.1039/C4CC08363F pmid: 33134246 |
|
|
(j) Peng, J.; Chen, C.; Xi, C. Chem. Sci. 2016, 7, 1383.
doi: 10.1039/C5SC03903G pmid: 33134246 |
|
|
(k) Sheng, J.; Wang, Y.; Chen, C. Angew. Chem., Int. Ed. 2017, 56, 4824.
doi: 10.1002/anie.201700696 pmid: 33134246 |
|
|
(l) Sheng, J.; Wu, C.; Chen, C. Org. Lett. 2018, 20, 4458.
doi: 10.1021/acs.orglett.8b01748 pmid: 33134246 |
|
|
(m) Lin, J.; Zhou, J.; Chen, C. Eur. J. Org. Chem. 2019, 34 ,5963.
pmid: 33134246 |
|
| [12] |
(a) Yan, T.; Chen, C.; Wen, L. Chin. J. Org. Chem. 2021, 41, 3660. (in Chinese)
doi: 10.6023/cjoc202104007 |
|
( 颜廷勋, 陈超, 文丽荣, 有机化学, 2021, 41, 3660.)
doi: 10.6023/cjoc202104007 |
|
|
(b) Niu, Y.; Qu, H.; Chen, C. Chin. Chem. Lett. 2022, 31, 1541.
|
|
| [13] |
The synthesis of fluoro-containing compounds developed by our group: (a) Cai, S.; Chen, C.; Sun, Z.; Xi, C. Chem. Commun. 2013, 49, 4552.
doi: 10.1039/c3cc41331d |
|
(b) Cao, C. K.; Tretyakov, E.; Chen, C. Green Synth. Catal. 2021, 2, 62.
|
|
| [14] |
(a) Maraswami, M.; Pankajakshan, S.; Chen, G. Org. Lett. 2017, 19, 4223.
doi: 10.1021/acs.orglett.7b01859 |
|
(b) Kovacs, S.; Stirling, A.; Novak, Z. Adv. Synth. Catal. 2017, 359, 527.
doi: 10.1002/adsc.201601136 |
| [1] | 孟宪强, 杨艺, 梁万洁, 王靖涛, 张荣葵, 刘会. 钯催化联烯胺区域选择性芳基酚氧化反应[J]. 有机化学, 2024, 44(1): 224-231. |
| [2] | 王兢睿, 冯永奎, 王能中, 黄年玉, 姚辉. 钯催化立体选择性合成硝基烷类β-碳糖苷[J]. 有机化学, 2023, 43(9): 3216-3225. |
| [3] | 王玉超, 刘晋彪, 何智涛. 钯催化共轭二烯的不对称氢官能团化[J]. 有机化学, 2023, 43(8): 2614-2627. |
| [4] | 向勋, 何照林, 董秀琴. 钯和手性磷酸协同催化高效构建手性分子的研究进展[J]. 有机化学, 2023, 43(3): 791-808. |
| [5] | 孙美娇, 谭晶, 谭玉, 彭进松, 陈春霞. 钯催化3-(2-氨基嘧啶-4-基)吲哚2位C—H键芳基化反应的研究[J]. 有机化学, 2023, 43(11): 3945-3959. |
| [6] | 熊威, 石斌, 姜烜, 陆良秋, 肖文精. 配体调控钯催化乙烯基环状碳酰胺和异氰酸酯的差异性转化[J]. 有机化学, 2023, 43(1): 265-273. |
| [7] | 龚诚, 唐剑, 徐飞, 李鹏杰, 王泽田, 张玉敏, 余国贤, 王亮. 近年来过渡金属催化吡啶酮/异喹啉酮的C—H活化反应研究进展[J]. 有机化学, 2022, 42(7): 1925-1949. |
| [8] | 石宇冰, 白文己, 母伟花, 李江平, 于嘉玮, 连冰. 钯催化C—H键官能团化形成C—X (X=O, N, F, I, ……)键的密度泛函理论研究进展[J]. 有机化学, 2022, 42(5): 1346-1374. |
| [9] | 李玉东, 李莹, 董亚楠, 夏春谷, 李跃辉. 锰催化的碳酸乙烯亚乙酯对喹唑啉酮的C—H烯丙基化[J]. 有机化学, 2022, 42(3): 847-853. |
| [10] | 陈宏超, 吴奕晨, 于洋, 王鹏. 钯催化的烯烃异构化反应[J]. 有机化学, 2022, 42(3): 742-757. |
| [11] | 杨新拓, 陈品红, 刘国生. 钯催化烯烃的不对称Aza-Wacker反应: 高效合成手性1,3-噁嗪烷-2-酮[J]. 有机化学, 2022, 42(10): 3382-3389. |
| [12] | 贾仕虎, 陈思元, 刘泽水, 程鸿刚, 周强辉. 钯/新型轴手性膦-烯配体催化的吲哚不对称烯丙基烷基化反应[J]. 有机化学, 2022, 42(10): 3373-3381. |
| [13] | 张洁明, 倪航, 吴起, 杨俊锋, 张俊良. 钯催化的磷、硫和硅中心手性化合物的合成进展[J]. 有机化学, 2022, 42(10): 3118-3128. |
| [14] | 韩阳, 姜为超, 张靖, 彭进松, 陈春霞. 可见光促进钯催化C—H键胺化反应合成咔唑醌衍生物的研究[J]. 有机化学, 2022, 42(1): 266-276. |
| [15] | 张涛, 李尚达, 周春林, 王新超, 张孟, 高泽众, 李纲. 以芳基碘为碘化试剂的苯酚衍生物的位点选择性C—H键碘化反应[J]. 有机化学, 2021, 41(9): 3511-3520. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||