有机化学 ›› 2023, Vol. 43 ›› Issue (11): 3945-3959.DOI: 10.6023/cjoc202304012 上一篇 下一篇
研究论文
孙美娇, 谭晶*( ), 谭玉(
), 谭玉( ), 彭进松*(
), 彭进松*( ), 陈春霞*(
), 陈春霞*( )
)
收稿日期:2023-04-09
修回日期:2023-05-24
发布日期:2023-07-05
基金资助:
Meijiao Sun, Jing Tan( ), Yu Tan(
), Yu Tan( ), Jinsong Peng(
), Jinsong Peng( ), Chunxia Chen(
), Chunxia Chen( )
)
Received:2023-04-09
Revised:2023-05-24
Published:2023-07-05
Contact:
E-mail: Supported by:文章分享
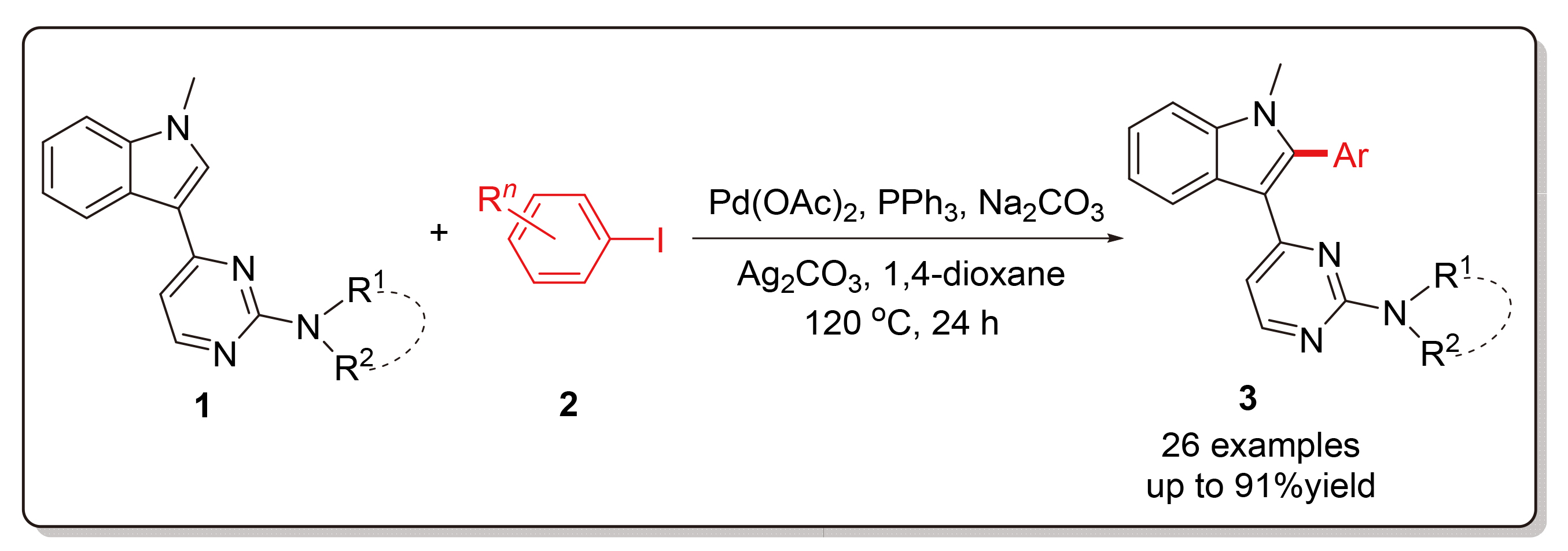
以N-甲基-3-(2-氨基嘧啶-4-基)吲哚衍生物和碘代芳烃为起始原料, 详细探讨了钯催化吲哚2位选择性C—H键活化/芳基化反应过程. 以醋酸钯为催化剂, 三苯基膦为配体, 碳酸银为添加剂, 碳酸钠为碱, 在120 ℃下于1,4-二氧六环中反应24 h, 可获得较高产率的2-芳基化吲哚衍生物. 在此标准条件下, 通过改变两种反应底物的结构, 探究了该合成方法的适用范围与局限性, 合成得到的系列吲哚衍生物经1H NMR、13C NMR和HRMS结构表征.
孙美娇, 谭晶, 谭玉, 彭进松, 陈春霞. 钯催化3-(2-氨基嘧啶-4-基)吲哚2位C—H键芳基化反应的研究[J]. 有机化学, 2023, 43(11): 3945-3959.
Meijiao Sun, Jing Tan, Yu Tan, Jinsong Peng, Chunxia Chen. Pd-Catalyzed C(2)—H Arylation of 3-(2-Aminopyrimidin-4-yl)indoles[J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3945-3959.
| Entry | Catalyst/Ligand | Additive | Base | Yieldb/% |
|---|---|---|---|---|
| 1 | Pd(OAc)2/Pyridine | Ag2CO3 | Na2CO3 | 52/57c |
| 2 | Pd(OAc)2/o-Phenanthroline | Ag2CO3 | Na2CO3 | Trace |
| 3 | Pd(OAc)2/Bipyridine | Ag2CO3 | Na2CO3 | Trace |
| 4 | Pd(OAc)2/PCy3 | Ag2CO3 | Na2CO3 | 12 |
| 5 | Pd(OAc)2/TFP | Ag2CO3 | Na2CO3 | 36 |
| 6 | Pd(OAc)2/ClPPh2 | Ag2CO3 | Na2CO3 | 11 |
| 7 | Pd(OAc)2/PPh3 | Ag2CO3 | Na2CO3 | 23/66c |
| 8 | Pd(OAc)2/P(o-tol)3 | Ag2CO3 | Na2CO3 | 8 |
| 9 | Pd(OAc)2/JohnPhosd | Ag2CO3 | Na2CO3 | Trace |
| 10 | Pd(OAc)2/CyJohnPhosd | Ag2CO3 | Na2CO3 | 16 |
| 11c | PdCl2/Pyridine | Ag2CO3 | Na2CO3 | 34 |
| 12c | Pd(TFA)2/Pyridine | Ag2CO3 | Na2CO3 | 11 |
| 13c | PdCl2(CH3CN)2/Pyridine | Ag2CO3 | Na2CO3 | 31 |
| 14c | PdCl2(PPh3)2/Pyridine | Ag2CO3 | Na2CO3 | 30 |
| 15c | Pd2(dba)3/Pyridine | Ag2CO3 | Na2CO3 | 50 |
| 16c | Pd(OAc)2/Pyridine | None | Na2CO3 | 4 |
| 17c,e | Pd(OAc)2/Pyridine | Ag2CO3 | Na2CO3 | 24 |
| 18c,e | Pd(OAc)2/Pyridine | Ag2SO4 | Na2CO3 | 19 |
| 19 | Pd(OAc)2/Pyridine | AgNO3 | Na2CO3 | Trace |
| 20 | Pd(OAc)2/Pyridine | AgSbF6 | Na2CO3 | 11 |
| 21 | Pd(OAc)2/Pyridine | AgOTf | Na2CO3 | 14 |
| 22f | Pd(OAc)2/PPh3 | Ag2CO3 | Na2CO3 | 76 |
| 23g | Pd(OAc)2/PPh3 | Ag2CO3 | Na2CO3 | 91 |
| 24g | Pd(OAc)2/PPh3 | Ag2CO3 | K2CO3 | 91 |
| 25g | Pd(OAc)2/PPh3 | Ag2CO3 | NaHCO3 | 85 |
| 26g | Pd(OAc)2/PPh3 | Ag2CO3 | NaOH | Trace |
| 27g | Pd(OAc)2/PPh3 | Ag2CO3 | KOH | 85 |
| 28g | Pd(OAc)2/PPh3 | Ag2CO3 | NaOtBu | 11 |
| 29g | Pd(OAc)2/PPh3 | Ag2CO3 | KOtBu | 35 |
| 30g | Pd(OAc)2/PPh3 | Ag2CO3 | Et3N | 61 |
| 31g | Pd(OAc)2 | Ag2CO3 | None | 5 |
| Entry | Catalyst/Ligand | Additive | Base | Yieldb/% |
|---|---|---|---|---|
| 1 | Pd(OAc)2/Pyridine | Ag2CO3 | Na2CO3 | 52/57c |
| 2 | Pd(OAc)2/o-Phenanthroline | Ag2CO3 | Na2CO3 | Trace |
| 3 | Pd(OAc)2/Bipyridine | Ag2CO3 | Na2CO3 | Trace |
| 4 | Pd(OAc)2/PCy3 | Ag2CO3 | Na2CO3 | 12 |
| 5 | Pd(OAc)2/TFP | Ag2CO3 | Na2CO3 | 36 |
| 6 | Pd(OAc)2/ClPPh2 | Ag2CO3 | Na2CO3 | 11 |
| 7 | Pd(OAc)2/PPh3 | Ag2CO3 | Na2CO3 | 23/66c |
| 8 | Pd(OAc)2/P(o-tol)3 | Ag2CO3 | Na2CO3 | 8 |
| 9 | Pd(OAc)2/JohnPhosd | Ag2CO3 | Na2CO3 | Trace |
| 10 | Pd(OAc)2/CyJohnPhosd | Ag2CO3 | Na2CO3 | 16 |
| 11c | PdCl2/Pyridine | Ag2CO3 | Na2CO3 | 34 |
| 12c | Pd(TFA)2/Pyridine | Ag2CO3 | Na2CO3 | 11 |
| 13c | PdCl2(CH3CN)2/Pyridine | Ag2CO3 | Na2CO3 | 31 |
| 14c | PdCl2(PPh3)2/Pyridine | Ag2CO3 | Na2CO3 | 30 |
| 15c | Pd2(dba)3/Pyridine | Ag2CO3 | Na2CO3 | 50 |
| 16c | Pd(OAc)2/Pyridine | None | Na2CO3 | 4 |
| 17c,e | Pd(OAc)2/Pyridine | Ag2CO3 | Na2CO3 | 24 |
| 18c,e | Pd(OAc)2/Pyridine | Ag2SO4 | Na2CO3 | 19 |
| 19 | Pd(OAc)2/Pyridine | AgNO3 | Na2CO3 | Trace |
| 20 | Pd(OAc)2/Pyridine | AgSbF6 | Na2CO3 | 11 |
| 21 | Pd(OAc)2/Pyridine | AgOTf | Na2CO3 | 14 |
| 22f | Pd(OAc)2/PPh3 | Ag2CO3 | Na2CO3 | 76 |
| 23g | Pd(OAc)2/PPh3 | Ag2CO3 | Na2CO3 | 91 |
| 24g | Pd(OAc)2/PPh3 | Ag2CO3 | K2CO3 | 91 |
| 25g | Pd(OAc)2/PPh3 | Ag2CO3 | NaHCO3 | 85 |
| 26g | Pd(OAc)2/PPh3 | Ag2CO3 | NaOH | Trace |
| 27g | Pd(OAc)2/PPh3 | Ag2CO3 | KOH | 85 |
| 28g | Pd(OAc)2/PPh3 | Ag2CO3 | NaOtBu | 11 |
| 29g | Pd(OAc)2/PPh3 | Ag2CO3 | KOtBu | 35 |
| 30g | Pd(OAc)2/PPh3 | Ag2CO3 | Et3N | 61 |
| 31g | Pd(OAc)2 | Ag2CO3 | None | 5 |
| Entry | S-1 | S-2 | Product 3 | Yieldb/% |
|---|---|---|---|---|
| 1 | | | | 90 |
| 2 | | | | 78 |
| 3 | | | | 34 |
| 4 | | | | 51 |
| 5 | | | | 70 |
| 6 | | | | 74 |
| 7 | | | | 77 |
| 8 | | | | 81 |
| 9 | | | | 88 |
| 10 | | | | 63 |
| 11 | | | | 66 |
| 12 | | | | 76 |
| 13 | | | | 63 |
| 14 | | | | 70 |
| 15 | | | | 65 |
| 16 | | | | 65 |
| 17 | | | | 55 |
| 18 | | | | 79 |
| 19 | | | | 51 |
| 20 | | | | 20 |
| 21 | | | | 21 |
| 22 | | | | 58 |
| 23 | | | | 44 |
| 24 | | | | 53 |
| 25 | | | | 7 8 |
| 26 | | | | 85 |
| Entry | S-1 | S-2 | Product 3 | Yieldb/% |
|---|---|---|---|---|
| 1 | | | | 90 |
| 2 | | | | 78 |
| 3 | | | | 34 |
| 4 | | | | 51 |
| 5 | | | | 70 |
| 6 | | | | 74 |
| 7 | | | | 77 |
| 8 | | | | 81 |
| 9 | | | | 88 |
| 10 | | | | 63 |
| 11 | | | | 66 |
| 12 | | | | 76 |
| 13 | | | | 63 |
| 14 | | | | 70 |
| 15 | | | | 65 |
| 16 | | | | 65 |
| 17 | | | | 55 |
| 18 | | | | 79 |
| 19 | | | | 51 |
| 20 | | | | 20 |
| 21 | | | | 21 |
| 22 | | | | 58 |
| 23 | | | | 44 |
| 24 | | | | 53 |
| 25 | | | | 7 8 |
| 26 | | | | 85 |
| [1] |
(a) Dorababu, A. RSC Med. Chem. 2020, 11, 1335.
doi: 10.1039/D0MD00288G |
|
(b) Han, Y.; Dong, W.; Guo, Q.; Li, X; Huang, L. Eur. J. Med. Chem. 2020, 203, 112506.
doi: 10.1016/j.ejmech.2020.112506 |
|
|
(c) Qin, H. L.; Liu, J.; Fang, W. Y.; Ravindar, L; Rakesh, K. P. Eur. J. Med. Chem. 2020, 194, 112245.
doi: 10.1016/j.ejmech.2020.112245 |
|
|
(d) Colenda, B. E.; Lee, H. S.; Reibenspies, J. H.; Hancock, R. D. Inorg. Chim. Acta. 2018, 482, 478.
doi: 10.1016/j.ica.2018.06.023 |
|
|
(e) Zhao, X. H.; Jia, Y. X.; Li, J. J.; Dong, R.H.; Zhang, J.J.; Ma, C. X.; Wang, H.; Rui, Y.K.; Jiang, X.Y. ACS Appl. Mater. Inter. 2018, 35, 29398.
|
|
|
(f) Mohbiya, D. R.; Sekar, N. Comput. Theor. Chem. 2018, 1139, 90.
doi: 10.1016/j.comptc.2018.07.015 |
|
|
(g) Zhao, J. F.; Dong, H.; Zheng, Y. J. J. Lumin. 2018, 195, 228.
doi: 10.1016/j.jlumin.2017.11.026 |
|
| [2] |
Zhang, M. Z.; Chen, Q.; Yang, G. F. Eur. J. Med. Chem. 2015, 89, 42.
doi: 10.1016/j.ejmech.2014.10.028 |
| [3] |
(a) Austin, O.; Chelsea, S.; Patrick, S.; Kent, A. C. ACS Med. Chem. Lett. 2018, 9, 901.
doi: 10.1021/acsmedchemlett.8b00212 pmid: 30048137 |
|
(b) Cong, H.; Zhao, X. H.; Castle, B. T.; Pomeroy, E. J.; Zhou, B.; John, L.; Wang, Y.; Bian, T. F.; Miao, Z. Y.; Zhang, W. N.; Sham, Y. Y.; David, J. O.; Craig, E. E.; Xing, C. G.; Zhuang, C. L. Mol. Pharmaceut. 2018, 15, 3892.
doi: 10.1021/acs.molpharmaceut.8b00359 pmid: 30048137 |
|
|
(c) Singh, P. K.; Silakari, O. Bioorg. Chem. 2018, 79, 163.
doi: 10.1016/j.bioorg.2018.04.001 pmid: 30048137 |
|
| [4] |
Kaushik, N. K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C. H.; Verma, A. K.; Choi, E. H. Molecules 2013, 18, 6620.
doi: 10.3390/molecules18066620 |
| [5] |
Ding, C. F.; Ma, H. X.; Yang, J.; Qin, X. J.; Guy, S. S.; Yu, H. F.; Wei, X.; Liu, Y. P.; Huang, W. Y.; Yang, Z. F.; Wang, X. H.; Luo, X. D.; Org. Lett. 2018, 20, 2702.
doi: 10.1021/acs.orglett.8b00913 |
| [6] |
(a) Zhang, Z.; Tanaka, K.; Yu, J. Q. Nature. 2017, 543, 538.
doi: 10.1038/nature21418 |
|
(b) Zhang, F. L.; Hong, K.; Li, T. J.; Yu, J. Q. Science 2016, 351, 252.
doi: 10.1126/science.aad7893 |
|
| [7] |
Dalpozzo, R.; Bartoli, G.; Bencivenni, G. Chem. Soc. Rev. 2012, 41, 7247.
doi: 10.1039/c2cs35100e pmid: 22899437 |
| [8] |
Kumari, A.; Singh, R. K. Bioorg. Chem. 2019, 89, 103021.
doi: 10.1016/j.bioorg.2019.103021 |
| [9] |
(a) Zhang, S. S.; Tan, Q. W.; Guan, L. P. Mini-Rev. Med. Chem. 2021, 21, 2285.
pmid: 30445859 |
|
(b) Okada, M.; Sugita, T.; Wong, C. P.; Wakimoto, T.; Abe, I. J. Nat. Prod. 2017, 80, 1205.
doi: 10.1021/acs.jnatprod.6b01152 pmid: 30445859 |
|
|
(c) Hong, W.; Li, J.; Chang, Z; Tan, X. L.; Yang, Y.; Ouyang, Y. F.; Yang, Y. H.; Kaur, S.; Paterson, I. C.; Ngeow, Y. F.; Wang, H. J. Antibiot. 2017, 70, 832.
doi: 10.1038/ja.2017.55 pmid: 30445859 |
|
|
(d) Kherkhache, H.; Benabdelaziz, I.; Silva, A. M. S.; Lahrech, M. B.; Benalia, M.; Haba, H. Nat. Prod. Res. 2020, 34, 1528.
doi: 10.1080/14786419.2018.1519817 pmid: 30445859 |
|
|
(e) Pulla, R. S.; Ummadi, N.; Gudi, Y.; Venkatapuram, P.; Adivireddy, P. J. Heterocyclic. Chem. 2018, 55, 115.
doi: 10.1002/jhet.v55.1 pmid: 30445859 |
|
| [10] |
(a) Esvan, Y. J.; Giraud, F.; Pereira, E.; Suchaud, V.; Nauton, L.; Théry, V.; Dezhenkova, L. G.; Kaluzhny, D. N.; Mazov, V. N.; Shtil, A. A.; Anizon, F.; Moreau, P. Bioorg. Med. Chem. 2016, 24, 3116.
doi: 10.1016/j.bmc.2016.05.032 |
|
(b) Karimabad, M. N.; Mahmoodi, M.; Jafarzadeh, A.; Darekordi, A.; Hajizadeh, M. R.; Hassanshahi, G. Mini-Rev. Med. Chem. 2019, 19, 540.
doi: 10.2174/1389557518666181116120145 |
|
| [11] |
Bian, M.; Wang, Z.; Xiong, X.; Sun, Y.; Matera, C.; Nicolaou, K. C.; Li, A. J. Am. Chem. Soc. 2012, 134, 8078.
doi: 10.1021/ja302765m |
| [12] |
Vasconcelos, S. N.; Meissner, K. A.; Ferraz, W. R.; Trossini, G. H.; Wrenger, C.; Stefani, H. A. Future Med. Chem. 2019, 11, 525.
doi: 10.4155/fmc-2018-0246 pmid: 30916995 |
| [13] |
Chadha, N.; Silakari, O. Eur. J. Med. Chem. 2017, 134, 159.
doi: 10.1016/j.ejmech.2017.04.003 |
| [14] |
Singh, P.; Prasher, P.; Dhillon, P.; Bhatti, R. Eur. J. Med. Chem. 2015, 97, 104.
doi: 10.1016/j.ejmech.2015.04.044 |
| [15] |
Sravanthi, T. V.; Manju, S. L. Eur. J. Pharm. Sci. 2016, 91, 1.
doi: 10.1016/j.ejps.2016.05.025 pmid: 27237590 |
| [16] |
Ye, Q. Y.; Xu, Y.; Zhao, J.; Gao, X. X; Chen, M. J.; Pan, R. l.; Zhong, W.; Wang, M. Z. Transl. Oncol. 2023, 31, 101637.
doi: 10.1016/j.tranon.2023.101637 |
| [17] |
Barnes, L.; Blaber, H.; Brooks, D. T. K.; Byers, L.; Buckley, D.; Byron, Z. C.; Chilvers, R. G.; Cochrane, L.; Cooney, E.; Damian, H. A.; Francis, L.; He, D. F.; Grace, J. M. J.; Green, H. J.; Hogarth, E. J. P.; Jusu, L.; Killalea, C. E.; King, O.; Lambert, J.; Lee, Z. J.; Lima, N. S.; Long, C. L.; Mackinnon, M.-L.; Mahdy, S.; Matthews-Wright, J.; Millward, M. J.; Meehan, M. F.; Merrett, C.; Morrison, L.; Parke, H. R. I.; Payne, C.; Payne, L.; Pike, C.; Seal, A.; Senior, A. J.; Smith, K. M.; Stanelyte, K.; Stillibrand, J.; Szpara, R.; Taday, F. F. H.; Threadgould, A. M.; Trainor, R. J.; Waters, J.; Williams, O.; Wong, C. K. W.; Wood, K.; Barton, N.; Gruszka, A.; Henley, Z.; Rowedder, J. E.; Cookson, R.; Jones, K. L.; Nadin, A.; Smith, I. E.; Macdonald, S. J. F.; Nortcliffe, A. J. Med. Chem. 2019, 62, 10402.
doi: 10.1021/acs.jmedchem.9b01499 pmid: 31647659 |
| [18] |
(a) Jian, H. H.; Xin, R. W.; Rui, N. D.; Xiao, Y. L.; Hong, M. L.; Tian, Y. Z.; Jun, Y. X.; Chen, H. L.; Yan, M.; Zhang, S. H.; Hou, W. F.; Tang, T. L.; Ya, D. C. J. Med. Chem. 2021, 64, 12548.
doi: 10.1021/acs.jmedchem.1c00271 |
|
(b) Yuan, S.; Wang, B.; Dai, Q. Q.; Zhang, X. N.; Zhang, J. Y.; Zuo, J. H.; Liu, H.; Chen, Z. S.; Li, G. B.; Wang, S. M.; Liu, H. M.; Yu, B. J. Med. Chem. 2021, 64, 14895.
doi: 10.1021/acs.jmedchem.1c01452 |
|
| [19] |
(a) Deng, H.; Jung, J.-K.; Liu, T.; Kuntz, K. W.; Snapper, M. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2003, 125, 9032.
doi: 10.1021/ja030249r pmid: 15469287 |
|
(b) Nicolaou, K. C.; Bheema Rao, P.; Hao, J.; Reddy, M. V.; Rassias, G.; Huang, X.; Chen, D. Y.; Snyder, S. A. Angew. Chem., Int. Ed. 2003, 42, 1753.
doi: 10.1002/anie.v42:15 pmid: 15469287 |
|
|
(c) Nicolaou, K. C.; Hao, J.; Reddy, M. V.; Rao, P. B.; Rassias, G.; Snyder, S. A.; Huang, X.; Chen, D. Y. K.; Brenzovich, W. E.; Giuseppone, N.; Giannakakou, P.; O'Brate, A. J. Am. Chem. Soc. 2004, 126, 12897.
pmid: 15469287 |
|
|
(d) Sitnikov, N.; Velder, J.; Abodo, L.; Cuvelier, N.; Neudorfl, J.; Prokop, A.; Krause, G.; Fedorov, A. Y.; Schmalz, H. G. Chem. - Eur. J. 2012, 18, 12096.
doi: 10.1002/chem.v18.38 pmid: 15469287 |
|
| [20] |
Kumar, P.; Nagtilak, P. J.; Kapur, M. New J. Chem. 2021, 45, 13692.
doi: 10.1039/D1NJ01696B |
| [21] |
(a) Chen, Z.; Wang, B.; Zhang, J.; Yu, W.; Liu, Z.; Zhang, Y. Org. Chem. Front. 2015, 2, 1107.
doi: 10.1039/C5QO00004A |
|
(b) Sandtorv, A. H. Adv. Synth. Catal. 2015, 357, 2403.
doi: 10.1002/adsc.v357.11 |
|
|
(c) Wen, J.; Shi, Z. Acc. Chem. Res. 2021, 54, 1723.
doi: 10.1021/acs.accounts.0c00888 |
|
|
(d) Luo, J. F.; Xu, X.; Zheng, J. L. Chin. J. Org. Chem. 2018, 38, 363. (in Chinese)
doi: 10.6023/cjoc201707031 |
|
|
(骆钧飞, 徐星, 郑俊良, 有机化学, 2018, 38, 363.)
doi: 10.6023/cjoc201707031 |
|
| [22] |
(a) Islam, S.; Larrosa, I. Chem. Eur. J. 2013, 19, 15093.
doi: 10.1002/chem.v19.45 pmid: 24959967 |
|
(b) Zheng, J.; Zhang, Y.; Cui, S. L. Org. Lett. 2014, 16, 3560.
doi: 10.1021/ol5014312 pmid: 24959967 |
|
|
(c) Miao, T.; Li, P.; Wang, G. W.; Wang, L. Chem. Asian J. 2013, 8, 3185.
doi: 10.1002/asia.v8.12 pmid: 24959967 |
|
| [23] |
(a) Lu, M.-Z.; Lu, P.; Xu, Y.-H.; Loh, T.-P. Org. Lett. 2014, 16, 2614.
doi: 10.1021/ol500754h |
|
(b) Lu, M.-Z.; Ding, X.; Shao, C. D.; Hu, Z. S.; Luo, H. Q.; Zhi, S. Z.; Hu, H. Y.; Kan, Y. H.; Loh, T.-P. Org. Lett. 2020, 22, 2663.
doi: 10.1021/acs.orglett.0c00631 |
|
|
(c) Tiwari, V. K.; Kamal, N.; Kapur, M. Org. Lett. 2015, 17, 1766.
doi: 10.1021/acs.orglett.5b00535 |
|
| [24] |
(a) Sun, P.; Yang, J. J.; MO, B. C.; Chen, X.; Li, X.; Chen, C. X. J. Org. Chem. 2020, 85, 6761.
doi: 10.1021/acs.joc.9b03153 pmid: 33251795 |
|
(b) Chen, X.; Sun, P.; MO, B. C.; Chen, C. X.; Peng, J. S. J. Org. Chem. 2021, 86, 352.
doi: 10.1021/acs.joc.0c02126 pmid: 33251795 |
|
|
(c) Li, X.; Chen, X.; Wang, H.; Chen, C. X.; Sun, P.; MO, B. C.; Peng, J. S. Org. Biomol. Chem. 2019, 17, 4014.
doi: 10.1039/C9OB00482C pmid: 33251795 |
|
|
(d) Li, X.; Bian, Y. Y.; Chen, X.; Zhang, H.; Wang, W.; Ren, S. D.; Yang, X. C.; Lu, C.; Chen, C. X.; Peng, J. S. Org. Biomol. Chem, 2019, 17, 321.
doi: 10.1039/C8OB02437E pmid: 33251795 |
|
|
(e) Yue, Y. X.; Peng, J. S.; Wang, D. Q.; Bian, Y. Y.; Sun, P.; Chen, C. X. J. Org. Chem. 2017, 82, 5481.
doi: 10.1021/acs.joc.7b00640 pmid: 33251795 |
|
| [25] |
Yanagisawa, S.; Itami, K. Tetrahedron. 2011, 67, 4425.
doi: 10.1016/j.tet.2011.03.093 |
| [26] |
(a) Mota, A. J.; Dedieu, A.; Bour, C.; Suffert, J. J. Am. Chem. Soc. 2005, 127, 7171.
doi: 10.1021/ja050453+ |
|
(b) Garcia-Cuadrado, D.; Braga, A. A. C.; Maseras, F.; Echavarren, A. M. J. Am. Chem. Soc. 2006, 128, 6798.
doi: 10.1021/ja061590p |
|
| [27] |
Jia, H. J.; Wu, K. Y.; Wang, Y. X. CN 108017620 2017 [Chem. Abstr. 2017, 10, 747229]
|
| [28] |
Zhang, X. X.; Chen, T. P.; Zhu, G. Y. CN 113563310 2021 [Chem. Abstr. 2021, 10, 709318]
|
| [1] | 孟宪强, 杨艺, 梁万洁, 王靖涛, 张荣葵, 刘会. 钯催化联烯胺区域选择性芳基酚氧化反应[J]. 有机化学, 2024, 44(1): 224-231. |
| [2] | 王兢睿, 冯永奎, 王能中, 黄年玉, 姚辉. 钯催化立体选择性合成硝基烷类β-碳糖苷[J]. 有机化学, 2023, 43(9): 3216-3225. |
| [3] | 王玉超, 刘晋彪, 何智涛. 钯催化共轭二烯的不对称氢官能团化[J]. 有机化学, 2023, 43(8): 2614-2627. |
| [4] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| [5] | 芦军, 李奇闯, 梁仁校, 贾义霞. 镍催化吡啶/喹啉鎓盐分子内去芳构化芳基加成反应[J]. 有机化学, 2023, 43(5): 1875-1882. |
| [6] | 向勋, 何照林, 董秀琴. 钯和手性磷酸协同催化高效构建手性分子的研究进展[J]. 有机化学, 2023, 43(3): 791-808. |
| [7] | 陈东平, 杨春红, 李明, 赵国孝, 王文鹏, 王喜存, 权正军. 芳炔参与的三组分芳基化反应进展[J]. 有机化学, 2023, 43(2): 503-525. |
| [8] | 马伟源, 戴惠芳, 亢少林, 张天麟, 舒兴中. 芳基乙烯基硅烷与芳基卤代物的Hiyama偶联反应[J]. 有机化学, 2023, 43(10): 3614-3622. |
| [9] | 熊威, 石斌, 姜烜, 陆良秋, 肖文精. 配体调控钯催化乙烯基环状碳酰胺和异氰酸酯的差异性转化[J]. 有机化学, 2023, 43(1): 265-273. |
| [10] | 贾雪锋, 仝向娟. 铜(II)配合物催化Chan-Lam偶联反应研究进展[J]. 有机化学, 2022, 42(9): 2640-2658. |
| [11] | 黄泽鑫, 尹宇强, 贾丰成, 吴安心. 吲哚及其衍生物C2—C3键断裂的反应研究进展[J]. 有机化学, 2022, 42(7): 2028-2044. |
| [12] | 曹成瑶, 牛亚茹, 蒋昀辰, 曲红梅, 陈超. 钯催化的氯二氟乙基高价碘试剂对于乙酰苯胺C—H键的氯二氟乙基化反应研究[J]. 有机化学, 2022, 42(7): 2098-2105. |
| [13] | 石宇冰, 白文己, 母伟花, 李江平, 于嘉玮, 连冰. 钯催化C—H键官能团化形成C—X (X=O, N, F, I, ……)键的密度泛函理论研究进展[J]. 有机化学, 2022, 42(5): 1346-1374. |
| [14] | 谢吴成, 陈浒, 黎韵越, 林洁玲, 陈婉雯, 石君君. 导向碳氢键活化与不饱和分子的电氧化环化反应[J]. 有机化学, 2022, 42(5): 1286-1306. |
| [15] | 刘文启, 沈振陆, 徐森苗. 三苯基砷/铱催化的非活化一级碳氢键的双硼化反应合成1,1-偕二硼烷[J]. 有机化学, 2022, 42(4): 1101-1110. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||