有机化学 ›› 2023, Vol. 43 ›› Issue (1): 338-344.DOI: 10.6023/cjoc202205029 上一篇 下一篇
研究简报
田冲a, 孙奇b, 王俊锋b, 陈俏a, 温志国a, Maxim Borzova, 聂万丽a,*( )
)
收稿日期:2022-05-18
修回日期:2022-07-20
发布日期:2022-08-25
通讯作者:
聂万丽
基金资助:
Chong Tiana, Qi Sunb, Junfeng Wangb, Qiao Chena, Zhiguo Wena, Maxim Borzova, Wanli Niea( )
)
Received:2022-05-18
Revised:2022-07-20
Published:2022-08-25
Contact:
Wanli Nie
Supported by:文章分享
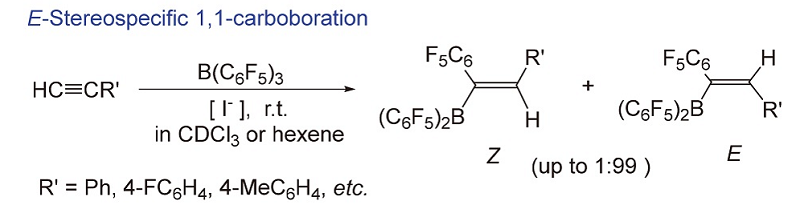
碳硼化反应作为有机合成双官能化反应的策略之一, 是合成各种天然产物、药物分子及精细化工产品重要的方法. 对卤素阴离子催化的立体可控炔烃碳硼化反应进行了详细研究, 通过卤素阴离子种类、用量、反应底物、溶剂、温度和时间等因素对反应活性、立体选择性的影响及其相互作用机制的探索, 发展了一种高立体选择性地制备E式1,1-碳硼化物的新方法; 相关研究表明, 不同的Z、E构型碳硼化物作为催化剂, 对环氧环己烷开环聚合的活性具有明显的差异. 开展相关的立体可控碳硼化反应新方法的研究, 不仅进一步拓展了烯基硼化物在有机双官能团转化反应中的应用, 也对发展新型含硼有机化合物的研究与应用具有重要意义.
田冲, 孙奇, 王俊锋, 陈俏, 温志国, Maxim Borzov, 聂万丽. 卤素阴离子催化的立体可控炔烃碳硼化反应研究[J]. 有机化学, 2023, 43(1): 338-344.
Chong Tian, Qi Sun, Junfeng Wang, Qiao Chen, Zhiguo Wen, Maxim Borzov, Wanli Nie. E-Stereospecific 1,1-Carboboration of Terminal Arylalkynes with [IB(C6F5)3]–[J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 338-344.
| Entry | Alkynes | [X–] (X∶BCF) | 2 (Z∶E)a |
|---|---|---|---|
| 1 | 1a | A (1∶1) | 2a (>1∶99) |
| 2 | 1a | B (1∶1) | 2a (>1∶99) |
| 3 | 1a | C (1∶1) | 2a (1∶1) |
| 4 | 1a | D (1∶1) | 2a (1∶1) |
| 5 | 1b | B (1∶1) | 2b (>1∶99) |
| 6 | 1c | B (1∶1) | 2c (>1∶99) |
| 7 | 1a | B (1∶5) | 2a (1∶10) |
| 8 | 1b | B (1∶5) | 2b (1∶17) |
| 9 | 1c | B (1∶5) | 2c (1∶22) |
| 10 | 1ab | B (1∶5) | 2a (>1∶99) |
| 11 | 1bb | B (1∶5) | 2b (>1∶99) |
| 12 | 1cb | B (1∶5) | 2c (>1∶99) |
| 13 | 1db | B (1∶5) | 2d (>1∶99) |
| 14 | 1eb | B (1∶5) | 2e (1∶14) |
| 15 | 1fb | B (1∶5) | 2f (1∶1) |
| 16 | 1gb | B (1∶5) | 2g (1∶3) |
| 17 | 1hb | B (1∶5) | 2h (1∶6) |
| 18 | 1ib | B (1∶5) | 2i (1∶3) |
| 19 | 1j | B (1∶1) | 2j (1∶1) |
| 20 | 1k | B (1∶1) | 2k (1∶3) |
| 21 | 1l | B (1∶1) | 2l (1∶4) |
| Entry | Alkynes | [X–] (X∶BCF) | 2 (Z∶E)a |
|---|---|---|---|
| 1 | 1a | A (1∶1) | 2a (>1∶99) |
| 2 | 1a | B (1∶1) | 2a (>1∶99) |
| 3 | 1a | C (1∶1) | 2a (1∶1) |
| 4 | 1a | D (1∶1) | 2a (1∶1) |
| 5 | 1b | B (1∶1) | 2b (>1∶99) |
| 6 | 1c | B (1∶1) | 2c (>1∶99) |
| 7 | 1a | B (1∶5) | 2a (1∶10) |
| 8 | 1b | B (1∶5) | 2b (1∶17) |
| 9 | 1c | B (1∶5) | 2c (1∶22) |
| 10 | 1ab | B (1∶5) | 2a (>1∶99) |
| 11 | 1bb | B (1∶5) | 2b (>1∶99) |
| 12 | 1cb | B (1∶5) | 2c (>1∶99) |
| 13 | 1db | B (1∶5) | 2d (>1∶99) |
| 14 | 1eb | B (1∶5) | 2e (1∶14) |
| 15 | 1fb | B (1∶5) | 2f (1∶1) |
| 16 | 1gb | B (1∶5) | 2g (1∶3) |
| 17 | 1hb | B (1∶5) | 2h (1∶6) |
| 18 | 1ib | B (1∶5) | 2i (1∶3) |
| 19 | 1j | B (1∶1) | 2j (1∶1) |
| 20 | 1k | B (1∶1) | 2k (1∶3) |
| 21 | 1l | B (1∶1) | 2l (1∶4) |
| Entry | [B] | [CHO]∶1 | Conv./% | Mn/(kg•mol–1) | PDI |
|---|---|---|---|---|---|
| 1 | BCF | 50000∶1 | 75.5 | 1.17 | 1.98 |
| 2 | (Z/E)-2a | 50000∶1 | 66.0 | 1.06 | 2.01 |
| 3 | (Z)-2a | 50000∶1 | 81.8 | 1.42 | 2.00 |
| 4 | (E)-2a | 5000∶1 | 71.4 | 4.91 | 2.63 |
| 5 | (Z/E)-2b | 50000∶1 | 75.7 | 1.37 | 1.90 |
| 6 | (Z)-2b | 50000∶1 | 77.8 | 1.42 | 2.07 |
| 7 | (E)-2b | 5000∶1 | 77.6 | 5.52 | 1.73 |
| Entry | [B] | [CHO]∶1 | Conv./% | Mn/(kg•mol–1) | PDI |
|---|---|---|---|---|---|
| 1 | BCF | 50000∶1 | 75.5 | 1.17 | 1.98 |
| 2 | (Z/E)-2a | 50000∶1 | 66.0 | 1.06 | 2.01 |
| 3 | (Z)-2a | 50000∶1 | 81.8 | 1.42 | 2.00 |
| 4 | (E)-2a | 5000∶1 | 71.4 | 4.91 | 2.63 |
| 5 | (Z/E)-2b | 50000∶1 | 75.7 | 1.37 | 1.90 |
| 6 | (Z)-2b | 50000∶1 | 77.8 | 1.42 | 2.07 |
| 7 | (E)-2b | 5000∶1 | 77.6 | 5.52 | 1.73 |
| [1] |
Ding, R.; Liu, Y.-G.; Han, M.-R.; Jiao, W.-Y.; Li, J.-Q.; Tian, Y.-H.; Sun, B.-G. J. Org. Chem. 2018, 83, 12939.
doi: 10.1021/acs.joc.8b02190 pmid: 30240220 |
| [2] |
Reddy, D.; Fronczek, F. R.; Watkins, E. B. Org. Lett. 2016, 18, 5620.
doi: 10.1021/acs.orglett.6b02848 |
| [3] |
Reddy, M. D.; Watkins, E. B. J. Org. Chem. 2015, 80, 11447.
doi: 10.1021/acs.joc.5b02138 |
| [4] |
Roy, S.; Roy, S.; Gribble, G. W. Tetrahedron 2012, 68, 9867.
doi: 10.1016/j.tet.2012.08.065 |
| [5] |
Luszczki, J. J.; Swiader, M. J.; Swiader, K.; Paruszewski, R.; Turski, W. A.; Czuczwar, S. J. Fund. Clin. Pharmacol. 2008, 22, 69.
|
| [6] |
Liu, Q.-C.; Liu, L.-L.; Ranjala, R.; Hendrik, L.; Guo, Y.; Ye, T. Chin. J. Chem. 2020, 38, 1280.
doi: 10.1002/cjoc.202000222 |
| [7] |
Bode, J. W.; Sohn, S. S. J. Am. Chem. Soc. 2007, 129, 13798.
doi: 10.1021/ja0768136 |
| [8] |
Gunanathan, C.; Ben-David, Y.; Milstein, D. Science 2007, 790.
|
| [9] |
Malawska, B. Curr. Top. Med. Chem. 2005, 5, 69.
pmid: 15638779 |
| [10] |
Shaabani, A.; Soleimani, E.; Rezayan, A. H. Tetrahedron Lett. 2007, 48, 6137.
doi: 10.1016/j.tetlet.2007.06.136 |
| [11] |
Kobayashi, I.; Muraoka, H.; Hasegawa, M.; Saika, T.; Nishida, M.; Kawamura, M.; Ando, R. J. Antimicrob. Chemother. 2002, 50, 129.
doi: 10.1093/jac/dkf106 |
| [12] |
Graul, A.; Castaner, J. Drugs Future 1997, 22, 956.
doi: 10.1358/dof.1997.022.09.423212 |
| [13] |
Merino, O.; Santoyo, B. M.; Montiel, L. E.; Jiménez-Vázquez, H. A.; Zepeda, L. G.; Tamariz, J. Tetrahedron Lett. 2010, 51, 3738.
doi: 10.1016/j.tetlet.2010.05.034 |
| [14] |
Najera, C.; Sansano, J. M. Chem. Rev. 2007, 107, 4584.
doi: 10.1021/cr050580o |
| [15] |
Tanaka, H.; Kuroda, A.; Marusawa, H.; Kino, T.; Goto, T.; Hashimoto, M.; Taga, T. J. Am. Chem. Soc. 1987, 109, 5031.
doi: 10.1021/ja00250a050 |
| [16] |
Montalban, A. G.; Boman, E.; Chang, C. D.; Ceide, S. C.; Dahl, R.; Dalesandro, D.; Delaet, N. G. J.; Erb, E.; Ernst, J. T.; Gibbs, A.; Kahl, J.; Kessler, L.; Kucharski, J.; Lum, C.; Lundstroem, J.; Miller, S.; Nakanishi, H.; Roberts, E.; Saiah, E.; Sullivan, R.; Urban, J.; Wang, Z.; Larson, C. J. Bioorg. Med. Chem. Lett. 2010, 20, 4819.
doi: 10.1016/j.bmcl.2010.06.102 pmid: 20663667 |
| [17] |
Ghosh, T.; Maity, P.; Ranu, B. J. Org. Chem. 2018, 83, 11758.
doi: 10.1021/acs.joc.8b01654 |
| [18] |
He, Z.-Y.; Guo, J.-Y.; Tian, S.-K. Adv. Synth. Catal. 2018, 360, 1544.
doi: 10.1002/adsc.201800012 |
| [19] |
Dong, H.; Hou, M.-F. Chin. J. Org. Chem. 2017, 37, 267. (in Chinese)
doi: 10.6023/cjoc201608014 |
|
(董浩, 侯梅芳, 有机化学, 2017, 37, 267.)
doi: 10.6023/cjoc201608014 |
|
| [20] |
Akerbladh, L.; Schembri, L. S.; Larhed, M.; Odell, L. R. J. Org. Chem. 2017, 82, 12520.
doi: 10.1021/acs.joc.7b02294 |
| [21] |
Mane, R. S.; Bhanage, B. M. J. Org. Chem. 2016, 81, 1223.
doi: 10.1021/acs.joc.5b02385 |
| [22] |
Sasaki, M.; Ando, M.; Kawahata, M.; Yamaguchi, K.; Takeda, K. Org. Lett. 2016, 18, 1598.
doi: 10.1021/acs.orglett.6b00455 |
| [23] |
Fan, W.-Z.; Shi, D.-Y.; Feng, B.-N. Tetrahedron Lett. 2015, 56, 4638.
doi: 10.1016/j.tetlet.2015.06.021 |
| [24] |
Giustiniano, M.; Mercalli, V.; Cassese, H.; Maro, S. D.; Galli, U.; Novellino, E.; Tron, G. C. J. Org. Chem. 2014, 79, 6006.
doi: 10.1021/jo5005444 |
| [25] |
Wang, Y.-Y.; Liu, Y.-Y. Acta Chim. Sinica 2019, 77, 418. (in Chinese)
doi: 10.6023/A19020061 |
|
(王昱赟, 刘云云, 化学学报, 2019, 77, 418.)
doi: 10.6023/A19020061 |
|
| [26] |
Liu, L.; Du, L.; Zhang, D.-N.; Du, Y.-F.; Zhao, K. Org. Lett. 2014, 16, 5772.
doi: 10.1021/ol502834g pmid: 25343425 |
| [27] |
Mupparapu, N.; Khan, S.; Battula, S.; Kushwaha, M.; Gupta, A. P.; Ahmed, Q. N.; Vishwakarma, R. A. Org. Lett. 2014, 16, 1152.
doi: 10.1021/ol5000204 pmid: 24490591 |
| [28] |
Cunico, R. F.; Chen, J.-X. Synth. Commun. 2003, 33, 1963.
doi: 10.1081/SCC-120020211 |
| [29] |
Yao, Y.; Li, W.-T.; Chen, J.-X. Chin. J. Org. Chem. 2014, 34, 2124. (in Chinese)
doi: 10.6023/cjoc201404048 |
|
(姚远, 李伟东, 陈建新, 有机化学, 2014, 34, 2124.)
doi: 10.6023/cjoc201404048 |
|
| [30] |
Yao, Y.; Tong, W.-T.; Chen, J.-X. Mendeleev Commun. 2014, 24, 176.
doi: 10.1016/j.mencom.2014.04.018 |
| [31] |
Chen, X.-J.; Chen, J.-X. Mendeleev Commun. 2013, 23, 106.
doi: 10.1016/j.mencom.2013.03.019 |
| [32] |
Cao, P.; Wen, X.-P.; Chen, J.-X. Synlett 2017, 28, 353.
doi: 10.1055/s-0036-1588346 |
| [33] |
Li, W.-D.; Han, S.-H.; Liu, Y.-H.; Chen, J.-X. Chin. J. Org. Chem. 2017, 37, 2423. (in Chinese)
doi: 10.6023/cjoc201703018 |
|
(李伟东, 韩生华, 刘艳红, 陈建新, 有机化学, 2017, 37, 2423.)
doi: 10.6023/cjoc201703018 |
|
| [34] |
Zhang, P.-P.; Chen, W.-W.; Feng, H.; Chen, J.-X. Chin. J. Org. Chem. 2019, 39, 3560. (in Chinese)
doi: 10.6023/cjoc201906033 |
|
(张鹏鹏, 陈雯雯, 冯花, 陈建新, 有机化学, 2019, 39, 3560.)
doi: 10.6023/cjoc201906033 |
|
| [35] |
Li, W.-D.; Han, Y.-L.; Chen, J.-X. Tetrahedron 2017, 73, 5813.
doi: 10.1016/j.tet.2017.08.035 |
| [36] |
Zhang, P.-P.; Han, S.-H.; Chen, J.-X. Chin. J. Org. Chem. 2020, 40, 1737. (in Chinese)
doi: 10.6023/cjoc202001020 |
|
(张鹏鹏, 韩生华, 陈建新, 有机化学, 2020, 40, 1737.)
doi: 10.6023/cjoc202001020 |
|
| [37] |
Liu, H.; Guo, Q.-L.; Chen, J.-X. Tetrahedron Lett. 2015, 56, 5747.
doi: 10.1016/j.tetlet.2015.09.022 |
| [38] |
Guo, Q.-L.; Wen, X.-P.; Chen, J.-X. Tetrahedron 2016, 72, 8117.
doi: 10.1016/j.tet.2016.10.066 |
| [39] |
Han, Y.-L.; Tong, W.-T.; Liu, H.; Chen, J.-X. Chin. J. Org. Chem. 2018, 38, 1993. (in Chinese)
doi: 10.6023/cjoc201803054 |
|
(韩宇玲, 仝文婷, 刘慧, 陈建新, 有机化学, 2018, 38, 1993.)
doi: 10.6023/cjoc201803054 |
|
| [40] |
Guo, Q. L.; Zhao, M. G.; Chen, J. X. Tetrahedron 2020, 76, 131476.
doi: 10.1016/j.tet.2020.131476 |
| [41] |
Tong, W.-T.; Cao, P.; Liu, Y.-H.; Chen, J.-X. J. Org. Chem. 2017, 82, 11603.
doi: 10.1021/acs.joc.7b01028 |
| [42] |
Wen, X.-P.; Chen, W. W.; Chen, J.-X. Appl.Organometal. Chem. 2019, 33, e5147.
|
| [43] |
Zhang, W.-J.; Cao, P.; Guo, Q.-L.; Chen, J.-X. Curr. Org. Synth. 2017, 14, 1067.
|
| [44] |
Zhang, W.- J; Han, S.-H.; Chen, J.-X. Synth. Commun. 2017, 47, 704.
doi: 10.1080/00397911.2017.1281958 |
| [45] |
Ma, F.; Liu, H.; Chen, J.-X. Tetrahedron Lett. 2016, 57, 5246.
doi: 10.1016/j.tetlet.2016.10.040 |
| [46] |
Han, Y. L.; Li, Y. P.; Han, S.-H.; Chen, J.-X. Synthesis 2019, 51, 2977.
doi: 10.1055/s-0037-1611778 |
| [47] |
Cunico, R. F.; Motta, A. R. Org. Lett. 2005, 7, 771.
doi: 10.1021/ol040066p |
| [48] |
Asahara, H.; Sofue, A.; Kuroda, Y.; Nishiwaki, N. J. Org. Chem. 2018, 83, 13691.
doi: 10.1021/acs.joc.8b01865 |
| [49] |
Weiner, B.; Szymanski, W.; Janssen, D. B.; Minnaard, A. J.; Feringa, B. L. Chem. Soc. Rev. 2010, 39, 1656.
doi: 10.1039/b919599h |
| [50] |
Cheng, R. P.; Gellman, S. H.; DeGrado, W. F. Chem. Rev. 2001, 101, 3219.
pmid: 11710070 |
| [51] |
Lelais, G.; Seebach, D. Biopolymers 2004, 76, 206.
doi: 10.1002/bip.20088 |
| [52] |
Vicario, J. L.; Badia, D.; Carrillo, L. Org. Lett. 2001, 3, 773.
pmid: 11259059 |
| [53] |
Liu, Y.-h.; Ha, Y.-L.; Chen, J.-X. Mendeleev Commun. 2021, 31, 128.
doi: 10.1016/j.mencom.2021.01.041 |
| [54] |
Fornicola, R. S.; Oblinger, E.; Montgomery, J. J. Org. Chem. 1998, 63, 3528.
doi: 10.1021/jo980477h |
| [55] |
Baichurin, R. I.; Baichurina, L. V.; Aboskalova, N. I.; Berestovitskaya, V. M. Russ. J. Gen. Chem. 2013, 83, 1764.
doi: 10.1134/S1070363213090223 |
| [56] |
Cativiela, C.; Ordonez, M.; Viveros-Ceballos, J. L. Tetrahedron 2020, 76, 130875.
doi: 10.1016/j.tet.2019.130875 |
| [57] |
Qian, X.-Y.; Xiong, P.; Xu, H.-C. Acta Chim. Sinica 2019, 77, 879. (in Chinese)
doi: 10.6023/A19050193 |
|
(钱向阳, 熊鹏, 徐海超, 化学学报, 2019, 77, 879.)
doi: 10.6023/A19050193 |
|
| [58] |
Han, Y.-Q.; Zhou, T. Chin. J. Chem. 2020, 38, 527.
doi: 10.1002/cjoc.202000041 |
| [59] |
Schollkopf, U.; Beckhaus, H. Angew. Chem., Int. Ed. Engl. 1976, 15, 293.
|
| [60] |
Cunico, R. F.; Pandey, R. K. J. Org. Chem. 2005, 70, 9048.
doi: 10.1021/jo0512406 |
| [1] | 李洋, 董亚楠, 李跃辉. 经由N-硼基酰胺中间体的酰胺高效转化合成腈类化合物[J]. 有机化学, 2024, 44(2): 638-643. |
| [2] | 付雅彤, 孙超凡, 张丹, 金成国, 陆居有. 巢式-碳硼烷硼氢键官能化反应研究进展[J]. 有机化学, 2024, 44(2): 438-447. |
| [3] | 贝文峰, 潘健, 冉冬梅, 刘伊琳, 杨震, 冯若昆. 基于钴催化吲哚酰胺与二炔和单炔的[4+2]环化反应合成γ-咔啉酮[J]. 有机化学, 2023, 43(9): 3226-3238. |
| [4] | 王兢睿, 冯永奎, 王能中, 黄年玉, 姚辉. 钯催化立体选择性合成硝基烷类β-碳糖苷[J]. 有机化学, 2023, 43(9): 3216-3225. |
| [5] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [6] | 卢凯, 屈浩琦, 陈樨, 秋慧, 郑晶, 马猛涛. 无催化剂、无溶剂条件下炔烃和烯烃与儿茶酚硼烷的硼氢化反应[J]. 有机化学, 2023, 43(6): 2197-2205. |
| [7] | 马佳敏, 李姣兄, 孟千森, 曾祥华. 炔烃的自由基砜基化反应研究进展[J]. 有机化学, 2023, 43(6): 2040-2052. |
| [8] | 陈志豪, 范奇, 尹标林, 李清江, 王洪根. α-硼取代羰基类化合物的合成进展[J]. 有机化学, 2023, 43(5): 1706-1712. |
| [9] | 刘双, 邹亮华, 王晓明. 均相钴催化氨硼烷的脱氢及转移氢化反应的研究进展[J]. 有机化学, 2023, 43(5): 1713-1725. |
| [10] | 蒋胜杰, 王杨, 徐信. 稀土金属配合物催化的甲胺基硼烷脱氢聚合反应[J]. 有机化学, 2023, 43(5): 1786-1791. |
| [11] | 李思达, 舒兴中, 吴立朋. 锆、钛介导的烯烃、炔烃硼氢化[J]. 有机化学, 2023, 43(5): 1751-1760. |
| [12] | 高师泉, 刘闯军, 杨俊锋, 张俊良. 钴催化的烯烃和炔烃的电化学还原偶联反应[J]. 有机化学, 2023, 43(4): 1559-1565. |
| [13] | 贾海瑞, 邱早早. 过渡金属催化硼-氢键活化合成含硼-杂原子键邻碳硼烷衍生物的研究进展[J]. 有机化学, 2023, 43(3): 1045-1068. |
| [14] | 向勋, 何照林, 董秀琴. 钯和手性磷酸协同催化高效构建手性分子的研究进展[J]. 有机化学, 2023, 43(3): 791-808. |
| [15] | 刘鹏, 钟富明, 廖礼豪, 谭伟强, 赵晓丹. 炔烃参与的去芳构化反应构建螺环己二烯酮类化合物的研究进展[J]. 有机化学, 2023, 43(12): 4019-4035. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||