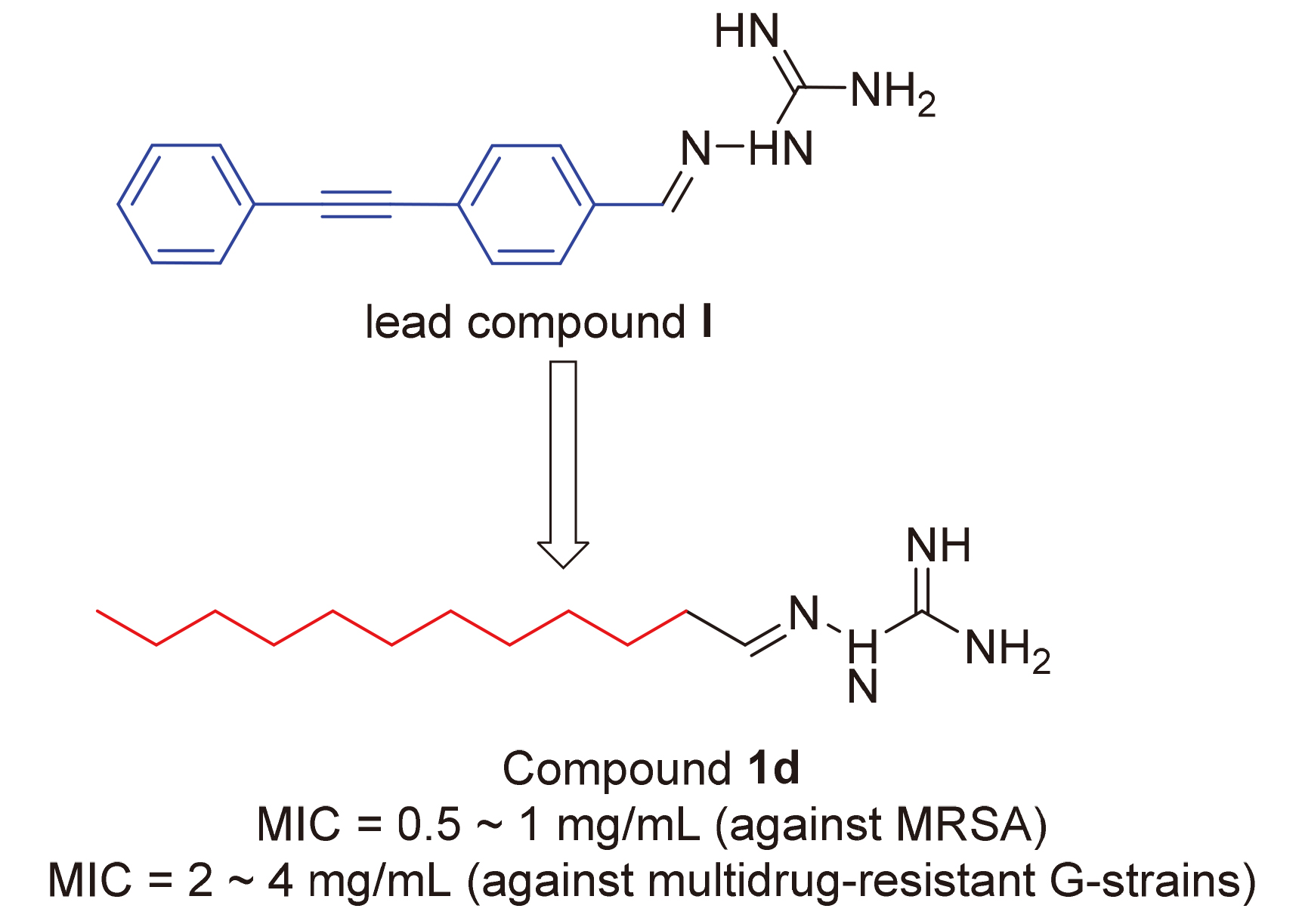
| [1] |
Hughes, D.; Andersson, D. I. Annu. Rev. Microbiol. 2017, 8, 579.
|
| [2] |
Mühlberg, E.; Umstätter, F.; Kleist, C.; Domhan, C.; Mier, W.; Uhl, P. Can. J. Microbiol. 2020, 66, 11.
doi: 10.1139/cjm-2019-0309
pmid: 31545906
|
| [3] |
Chellat, M. F.; Raguž, L.; Riedl, R. Angew. Chem., nt. Ed. 2016, 55, 6600.
|
| [4] |
Yang, T.; Wang, J. Y.; Cao, J. Y.; Zhang, X. Y.; Lai, Y.; Li, L. N.; Ye, X. Y.; You, C. Ital. J. Pediatr. 2021, 47, 169.
doi: 10.1186/s13052-021-01120-6
pmid: 34362428
|
| [5] |
So, M.; Walti, L. Curr. Infect. Dis. Rep. 2022, 24, 63.
doi: 10.1007/s11908-022-00778-1
|
| [6] |
Rai, M.; Zimowska, B.; Gade, A.; Ingle, P. AMB Express 2022, 12, 60.
doi: 10.1186/s13568-022-01404-y
|
| [7] |
Prasad, N. K.; Seiple, I. B.; Cirz, R. T.; Rosenberg, O. S. Antimicrob. Agents Chemother. 2022, 66, e0005422.
doi: 10.1128/aac.00054-22
|
| [8] |
Alfei, S.; Schito, A. M. Pharmaceuticals (Basel) 2022, 15, 476.
doi: 10.3390/ph15040476
|
| [9] |
Whittington, D. A.; Rusche, K. M.; Shin, H.; Fierke, C. A.; Christianson, D. W. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 8146.
doi: 10.1073/pnas.1432990100
|
| [10] |
Kalinin, D. V.; Holl, R. Curr. Top Med. Chem. 2016, 16, 2379.
doi: 10.2174/1568026616666160413135835
|
| [11] |
Erwin, A. L. Cold Spring Harbor Perspect. Med. 2016, 6, a025304.
doi: 10.1101/cshperspect.a025304
|
| [12] |
Zhou, P.; Hong, J. Acc. Chem. Res. 2021, 54, 1623.
doi: 10.1021/acs.accounts.0c00880
|
| [13] |
Kang, Y.; Zhao, M.; Zhang, J. Chin. J. Antibiot. 2017, 42, 169. (in Chinese)
|
|
(康悦, 赵明, 张菁, 中国抗生素杂志, 2017, 42, 169.)
|
| [14] |
Sidoryk, K.; Świtalska, M.; Rózga, P.; Wietrzyk, J.; Bujak, I.; Żerek, B.; Kaczmarek, Ł.; Cybulski, M. Med. Chem. Res. 2017, 26, 3354.
doi: 10.1007/s00044-017-2028-1
|
| [15] |
Dömötör, O.; May, N. V.; Gál, G. T.; Spengler, G.; Dobrova, A.; Arion, V. B.; Enyedy, É. A. Molecules 2022, 27, 2044.
doi: 10.3390/molecules27072044
|
| [16] |
Deng, X. Q.; Song, M. X. J. Enzyme Inhib. Med. Chem. 2020, 35, 354.
doi: 10.1080/14756366.2019.1702654
|
| [17] |
Yu, H.-H.; Zhou, S.-C.; Guo, T.-T.; Liang, Z.; Chen, H.-B.; Dai, W.-K.; Song, M.-X. Chin. J. Org. Chem. 2019, 39, 1497. (in Chinese)
doi: 10.6023/cjoc201811012
|
|
(余海红, 周胜超, 郭婷婷, 梁焯, 陈华斌, 代卫凯, 宋明霞, 有机化学, 2019, 39, 1497.)
doi: 10.6023/cjoc201811012
|
| [18] |
Song, M. X.; Wang, S. B.; Wang, Z. T.; Fu, Z. Y.; Zhou, S. C.; Cheng, H. B.; Liang, Z.; Deng, X. Q. Eur. J. Med. Chem. 2019, 166, 108.
doi: 10.1016/j.ejmech.2019.01.038
|
| [19] |
Brown, E. D.; Wright, G. D. Nature 2016, 529, 336.
doi: 10.1038/nature17042
|
| [20] |
Dubey, K. K.; Sharma, I. M. Arch. Pharm. (Weinheim, Ger.) 2020, 353, e2000168.
|
| [21] |
Makovitzki, A.; Avrahami, D.; Shai, Y. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 15997.
doi: 10.1073/pnas.0606129103
|
| [22] |
Hoque, J.; Akkapeddi, P.; Yadav, V.; Manjunath, G. B.; Uppu, D. S.; Konai, M. M.; Yarlagadda, V.; Sanyal, K.; Haldar, J. ACS Appl. Mater. Interfaces 2015, 7, 1804.
doi: 10.1021/am507482y
|
| [23] |
Kosowska-Shick, K.; Clark, C.; Pankuch, G. A.; McGhee, P.; Dewasse, B.; Beachel, L.; Appelbaum, P. C. Antimicrob. Agents Chemother. 2009, 53, 4217.
doi: 10.1128/AAC.00742-09
pmid: 19620338
|
| [24] |
Qiu, X.; Janson, C. A.; Smith, W. W.; Head, M.; Lonsdale, J.; Konstantinidis, A. K. J. Mol. Biol. 2001, 307, 341.
pmid: 11243824
|
 ), 黄玉珊b,*(
), 黄玉珊b,*( )
)
 ), Yushan Huangb,*(
), Yushan Huangb,*( )
)