有机化学 ›› 2023, Vol. 43 ›› Issue (6): 1991-2001.DOI: 10.6023/cjoc202211024 上一篇 下一篇
综述与进展
李格非a,b, 荆杰a, 罗振扬a, 默娟b, 熊德彩b, 叶新山b,*( )
)
收稿日期:2022-11-22
修回日期:2022-12-26
发布日期:2023-01-11
基金资助:
Gefei Lia,b, Jie Jinga, Zhenyang Luoa, Juan Mob, Decai Xiongb, Xinshan Yeb,*( )
)
Received:2022-11-22
Revised:2022-12-26
Published:2023-01-11
Contact:
E-mail: Supported by:文章分享

糖类在很多生命活动中发挥着重要的作用, 例如细胞壁组成、能量储存以及生物识别等. 发展高区域选择性和立体选择性的糖基化反应是目前糖化学研究的中心问题. 传统的化学糖基化方法往往需要依靠正交的保护基化学来调控糖基化反应的选择性. 近年来一些无保护糖基化的策略被开发出来, 可以在更简单、更温和的条件下实现寡糖及其缀合物的选择性合成. 对近期发展的无保护糖基化策略进行了综述, 并对无保护糖基化反应的未来发展前景进行展望.
李格非, 荆杰, 罗振扬, 默娟, 熊德彩, 叶新山. 无保护糖基化反应研究进展[J]. 有机化学, 2023, 43(6): 1991-2001.
Gefei Li, Jie Jing, Zhenyang Luo, Juan Mo, Decai Xiong, Xinshan Ye. Recent Advances in Protection-Free Glycosylations[J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1991-2001.
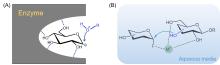
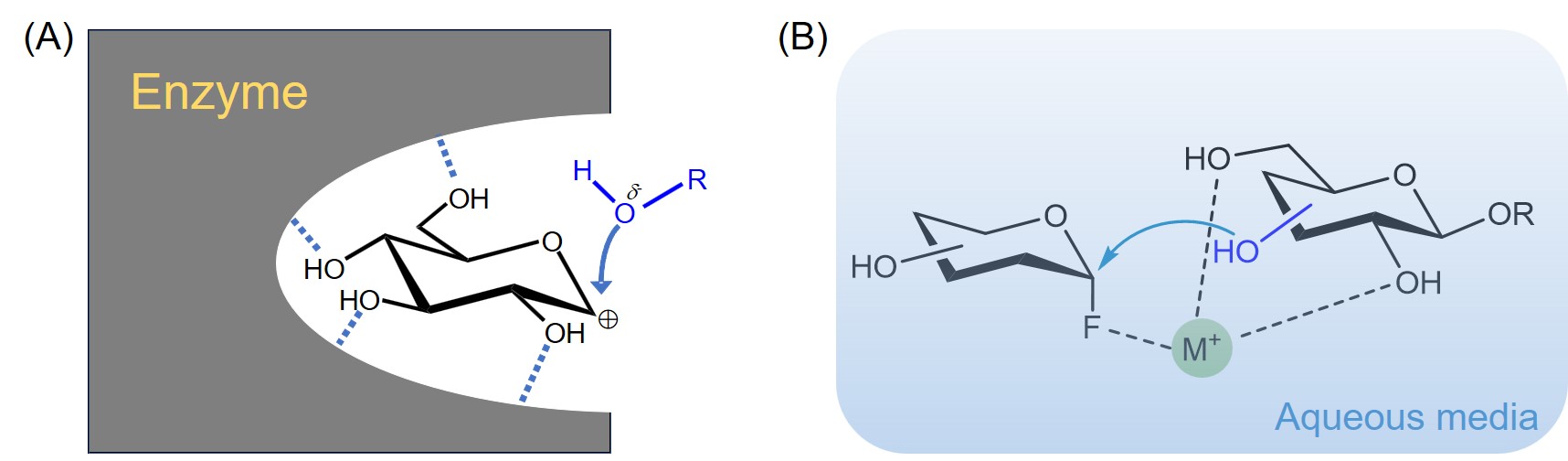
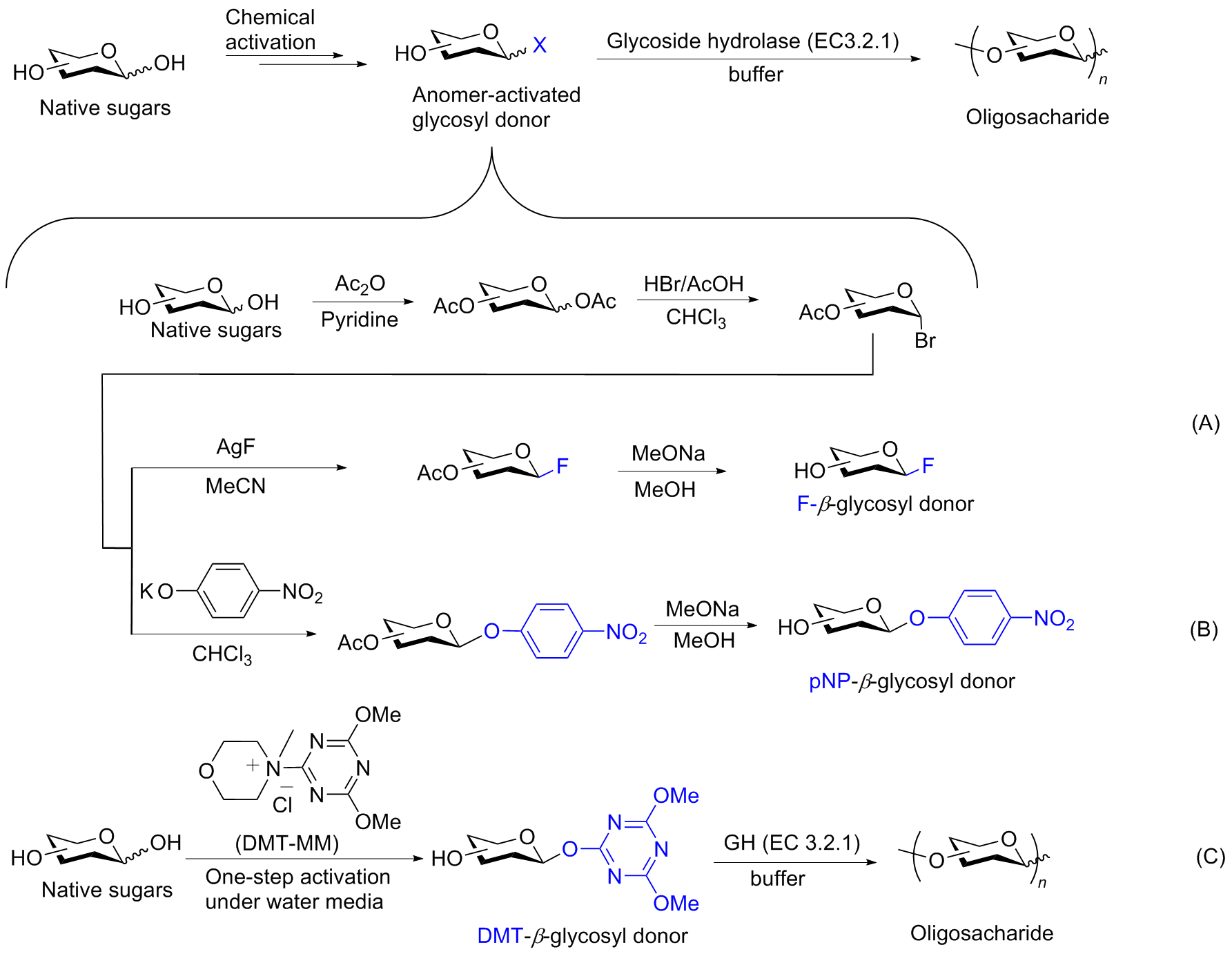
| [1] |
Clausen, T. M.; Sandoval, D. R.; Spliid, C. B.; Pihl, J.; Perrett, H. R.; Painter, C. D.; Narayanan, A.; Majowicz, S. A.; Kwong, E. M.; McVicar, R. N.; Thacker, B. E.; Glass, C. A.; Yang, Z.; Torres, J. L.; Golden, G. J.; Bartels, P. L.; Porell, R. N.; Garretson, A. F.; Laubach, L.; Feldman, J.; Yin, X.; Pu, Y.; Hauser, B. M.; Caradonna, T. M.; Kellman, B. P.; Martino, C.; Gordts, P.; Chanda, S. K.; Schmidt, A. G.; Godula, K.; Leibel, S. L.; Jose, J.; Corbett, K. D.; Ward, A. B.; Carlin, A. F.; Esko, J. D. Cell 2020, 183, 1043.
doi: 10.1016/j.cell.2020.09.033 pmid: 32970989 |
| [2] |
Moradi, S. V.; Hussein, W. M.; Varamini, P.; Simerska, P.; Toth, I. Chem. Sci. 2016, 7, 2492.
doi: 10.1039/C5SC04392A |
| [3] |
Qin, X.; Ye, X.-S. Chin. J. Chem. 2021, 39, 531.
doi: 10.1002/cjoc.v39.3 |
| [4] |
Yu, B. Acc. Chem. Res. 2018, 51, 507.
doi: 10.1021/acs.accounts.7b00573 |
| [5] |
Guo, J.; Ye, X.-S. Molecules 2010, 15, 7235.
doi: 10.3390/molecules15107235 |
| [6] |
Villadsen, K.; Martos-Maldonado, M. C.; Jensen, K. J.; Thygesen, M. B. ChemBioChem 2017, 18, 574.
doi: 10.1002/cbic.201600582 pmid: 28067438 |
| [7] |
Bojarova, P.; Rosencrantz, R. R.; Elling, L.; Kren, V. Chem. Soc. Rev. 2013, 42, 4774.
doi: 10.1039/c2cs35395d |
| [8] |
Mukaiyama, T.; Murai, Y.; Shoda, S. Chem. Lett. 1981, 10, 431.
doi: 10.1246/cl.1981.431 |
| [9] |
Nicolaou, K. C.; Dolle, R. E.; Papahatjis, D. P. J. Am. Chem. Soc. 1984, 106, 4189.
doi: 10.1021/ja00327a021 |
| [10] |
Nicolaou, K. C.; Caulfield, T. J.; Kataoka, H.; Stylianides, N. A. J. Am. Chem. Soc. 1990, 112, 3693.
doi: 10.1021/ja00165a084 |
| [11] |
Kobayashi, S.; Kashiwa, K.; Kawasaki, T.; Shoda, S. J. Am. Chem. Soc. 1991, 113, 3079.
doi: 10.1021/ja00008a042 |
| [12] |
Banait, N. S.; Jencks, W. P. J. Am. Chem. Soc. 1991, 113, 7951.
doi: 10.1021/ja00021a021 |
| [13] |
Banait, N. S.; Jencks, W. P. J. Am. Chem. Soc. 1991, 113, 7958.
doi: 10.1021/ja00021a022 |
| [14] |
Pelletier, G.; Zwicker, A.; Allen, C. L.; Schepartz, A.; Miller, S. J. J. Am. Chem. Soc. 2016, 138, 3175.
doi: 10.1021/jacs.5b13384 |
| [15] |
Wadzinski, T. J.; Steinauer, A.; Hie, L.; Pelletier, G.; Schepartz, A.; Miller, S. J. Nat. Chem. 2018, 10, 644.
doi: 10.1038/s41557-018-0041-8 pmid: 29713033 |
| [16] |
Wen, P.; Jia, P.; Fan, Q.; McCarty, B. J.; Tang, W. ChemSusChem 2022, 15, e202102483.
|
| [17] |
Zhang, G. L.; Gadi, M. R.; Cui, X.; Liu, D.; Zhang, J.; Saikam, V.; Gibbons, C.; Wang, P. G.; Li, L. Green Chem. 2021, 23, 2907.
doi: 10.1039/D1GC00098E |
| [18] |
Takahashi, D.; Tanaka, M.; Toshima, K. Trends Glycosci. Glyc. 2018, 30, E55.
doi: 10.4052/tigg.1817.2E |
| [19] |
Oshima, K.; Kitazono, E.-i.; Aoyama, Y. Tetrahedron Lett. 1997, 38, 5001.
doi: 10.1016/S0040-4039(97)01070-8 |
| [20] |
Oshima, K.; Aoyama, Y. J. Am. Chem. Soc. 1999, 121, 2315.
doi: 10.1021/ja982395g |
| [21] |
Gouliaras, C.; Lee, D.; Chan, L.; Taylor, M. S. J. Am. Chem. Soc. 2011, 133, 13926.
doi: 10.1021/ja2062715 |
| [22] |
Nakagawa, A.; Tanaka, M.; Hanamura, S.; Takahashi, D.; Toshima, K. Angew. Chem., Int. Ed. 2015, 54, 10935.
doi: 10.1002/anie.201504182 |
| [23] |
Tanaka, M.; Nakagawa, A.; Nishi, N.; Iijima, K.; Sawa, R.; Takahashi, D.; Toshima, K. J. Am. Chem. Soc. 2018, 140, 3644.
doi: 10.1021/jacs.7b12108 |
| [24] |
Tanaka, M.; Nashida, J.; Takahashi, D.; Toshima, K. Org. Lett. 2016, 18, 2288.
doi: 10.1021/acs.orglett.6b00926 |
| [25] |
Shoda, S.; Uyama, H.; Kadokawa, J.; Kimura, S.; Kobayashi, S. Chem. Rev. 2016, 116, 2307.
doi: 10.1021/acs.chemrev.5b00472 |
| [26] |
Shoda, S. Proc. Jpn. Acad. Ser. B 2017, 93, 125.
doi: 10.2183/pjab.93.008 |
| [27] |
Tanaka, T.; Noguchi, M.; Kobayashi, A.; Shoda, S. Chem. Comm. 2008, 17, 2016.
|
| [28] |
Kobayashi, A.; Tanaka, T.; Watanabe, K.; Ishihara, M.; Noguchi, M.; Okada, H.; Morikawa, Y.; Shoda, S. Bioorg. Med. Chem. Lett. 2010, 20, 3588.
doi: 10.1016/j.bmcl.2010.04.122 pmid: 20529686 |
| [29] |
Tanaka, T.; Noguchi, M.; Watanabe, K.; Misawa, T.; Ishihara, M.; Kobayashi, A.; Shoda, S. Org. Biomol. Chem. 2010, 8, 5126.
doi: 10.1039/c0ob00190b |
| [30] |
Noguchi, M.; Nakamura, M.; Ohno, A.; Tanaka, T.; Kobayashi, A.; Ishihara, M.; Fujita, M.; Tsuchida, A.; Mizuno, M.; Shoda, S. Chem. Commun. 2012, 48, 5560.
doi: 10.1039/c2cc30946g |
| [31] |
Tanaka, T.; Matsuura, A.; Aso, Y.; Ohara, H. J. Appl. Glycosci. 2020, 67, 119.
doi: 10.5458/jag.jag.JAG-2020_0010 |
| [32] |
Fujimoto, Y.; Mitsunobe, K.; Fujiwara, S.; Mori, M.; Hashimoto, M.; Suda, Y.; Kusumoto, S.; Fukase, K. Org. Biomol. Chem. 2013, 11, 5034.
doi: 10.1039/c3ob40899j pmid: 23804153 |
| [33] |
Tanaka, T.; Kikuta, N.; Kimura, Y.; Shoda, S. Chem. Lett. 2015, 44, 846.
doi: 10.1246/cl.150201 |
| [34] |
Ishihara, M.; Takagi, Y.; Li, G.; Noguchi, M.; Shoda, S. Chem. Lett. 2013, 42, 1235.
doi: 10.1246/cl.130646 |
| [35] |
Li, G.; Luo, Y.; Mo, J.; Noguchi, M.; Jing, J.; Luo, Z.; Shoda, S.; Ye, X.-S. Chin. Chem. Lett. 2023, 34, 107754.
doi: 10.1016/j.cclet.2022.107754 |
| [36] |
Noguchi, M.; Tanaka, T.; Gyakushi, H.; Kobayashi, A.; Shoda, S. J. Org. Chem. 2009, 74, 2210.
doi: 10.1021/jo8024708 pmid: 19203234 |
| [37] |
Li, G.; Noguchi, M.; Serizawa, K.; Shoda, S. Chimia 2018, 72, 874.
doi: 10.2533/chimia.2018.874 |
| [38] |
Fairbanks, A. J. Carbohydr. Res. 2021, 499, 108197.
doi: 10.1016/j.carres.2020.108197 |
| [39] |
Li, C.; Wang, L.-X. Chem. Rev. 2018, 118, 8359.
doi: 10.1021/acs.chemrev.8b00238 |
| [40] |
Huang, W.; Giddens, J.; Fan, S. Q.; Toonstra, C.; Wang, L.-X. J. Am. Chem. Soc. 2012, 134, 12308.
doi: 10.1021/ja3051266 pmid: 22747414 |
| [41] |
Tanaka, T.; Nagai, H.; Noguchi, M.; Kobayashi, A.; Shoda, S. Chem. Commun. 2009, 45, 3378.
|
| [42] |
Li, G.; Ma, W.; Mo, J.; Cheng, B.; Shoda, S.; Zhou, D.; Ye, X.-S. ACS Appl. Mater. Interfaces 2021, 13, 46260.
doi: 10.1021/acsami.1c11561 |
| [43] |
Meguro, Y.; Noguchi, M.; Li, G.; Shoda, S. Org. Lett. 2018, 20, 76.
doi: 10.1021/acs.orglett.7b03400 |
| [44] |
Meguro, Y.; Noguchi, M.; Li, G.; Shoda, S. Tetrahedron Lett. 2020, 61, 152198.
doi: 10.1016/j.tetlet.2020.152198 |
| [45] |
Li, G.; Dao, Y.; Mo, J.; Dong, S.; Shoda, S.; Ye, X.-S. CCS Chem. 2022, 4, 1930.
doi: 10.31635/ccschem.021.202101115 |
| [46] |
Li, G.; Noguchi, M.; Kashiwagura, H.; Tanaka, Y.; Serizawa, K.; Shoda, S. Tetrahedron Lett. 2016, 57, 3529.
|
| [47] |
Li, G.; Noguchi, M.; Nakamura, K.; Hayasaka, R.; Tanaka, Y.; Shoda, S. Tetrahedron Lett. 2018, 59, 3428.
doi: 10.1016/j.tetlet.2018.08.005 |
| [48] |
Li, G.; Noguchi, M.; Arisaka, G.; Tanaka, Y.; Shoda, S. Org. Biomol. Chem. 2021, 19, 3134.
doi: 10.1039/D1OB00311A |
| [49] |
Sinnott, M. L. Chem. Rev. 1990, 90, 1171.
doi: 10.1021/cr00105a006 |
| [50] |
Qiu, X.; Fairbanks, A. J. Org. Biomol. Chem. 2020, 18, 7355.
doi: 10.1039/D0OB01727B |
| [51] |
Guchhait, G.; Misra, A. K. Catal. Comm. 2011, 14, 52.
doi: 10.1016/j.catcom.2011.07.016 |
| [52] |
Gorityala, B. K.; Ma, J.; Pasunooti, K. K.; Cai, S.; Liu, X. Green Chem. 2011, 13, 573.
doi: 10.1039/c0gc00883d |
| [53] |
Javier Muñoz, F.; André, S.; Gabius, H.-J.; Sinisterra, J. V.; Hernáiz, M. J.; Linhardt, R. J. Green Chem. 2009, 11, 373.
doi: 10.1039/B814171A |
| [54] |
Schmanlisch, S.; Mahrwald, R. Org. Lett. 2013, 15, 5854.
doi: 10.1021/ol402914v |
| [55] |
Izumi, M.; Fukase, K.; Kusumoto, S. Biosci. Biotechnol. Biochem. 2002, 66, 211.
doi: 10.1271/bbb.66.211 |
| [56] |
Shaikh, N.; Russo, L.; Cipolla, L.; Nicotra, F. Mol. Diversity 2011, 15, 341.
doi: 10.1007/s11030-010-9281-2 |
| [57] |
Mamidyala, S. K.; Finn, M. G. J. Org. Chem. 2009, 74, 8417.
doi: 10.1021/jo901857x pmid: 19827757 |
| [58] |
Meng, B.; Zhu, Z.; Baker, D. C. Org. Biomol. Chem. 2014, 12, 5182.
doi: 10.1039/c4ob00626g pmid: 24915049 |
| [59] |
Gudmundsdottir, A. V.; Nitz, M. Org. Lett. 2008, 10, 3461.
doi: 10.1021/ol801232f pmid: 18616337 |
| [60] |
Williams, R. J.; Paul, C. E.; Nitz, M. Carbohydr. Res. 2014, 386, 73.
doi: 10.1016/j.carres.2013.08.019 |
| [61] |
Edgar, L. J. G.; Dasgupta, S.; Nitz M. Org. Lett. 2012, 14, 4226.
doi: 10.1021/ol3019083 |
| [62] |
Alexander, S. R.; Fairbanks, A. J. Org. Biomol. Chem. 2016, 14, 6679.
doi: 10.1039/c6ob01069e pmid: 27327112 |
| [63] |
Wan, L.; Zhang, X.; Zou, Y.; Shi, R.; Cao, J.; Xu, S.; Deng, L.; Zhou, L.; Gong, Y.; Shu, X.; Lee, G.-Y.; Ren, H.; Dai, L.; Qi, S.; Houk, K.-N.; Niu, D. J. Am. Chem. Soc. 2021, 143, 11919.
doi: 10.1021/jacs.1c05156 |
| [64] |
Takeuchi, H.; Fujimori, Y.; Uedda, Y.; Shibayama, H.; Nagaishi, M.; Yoshimura, T.; Sasamori, T.; Tokitoh, N.; Furuta, T.; Kawabata, T. Org. Lett. 2020, 22, 4754.
doi: 10.1021/acs.orglett.0c01549 |
| [65] |
Yao, W.; Xiong, D.-C.; Yang, Y.; Geng, C.; Cong, Z.; Li, F.; Li, B.-H.; Qin, X.; Wang, L-N.; Xue, W.-Y.; Yu, N.; Zhang, H.; Wu, X.; Liu, M.; Ye, X.-S. Nat. Synth. 2020, 854.
|
| [1] | 李文艺, 唐胤恒, 欧阳文韬, 陆雨函, 陈锦杨, 何卫民. N-无保护苯胺电化学硒醚化反应构建4-(烃基硒代)苯胺[J]. 有机化学, 2021, 41(12): 4766-4772. |
| [2] | 颜世强, 张伟, 丁宁, 李英霞 . 硅胶及其负载酸在糖化学中的应用研究进展[J]. 有机化学, 2012, 32(11): 2081-2089. |
| [3] | 宋秀美, 谭越河, 李建晓, 汪朝阳. 无保护α-氨基酸合成多手性中心2(5H)-呋喃酮化合物[J]. 有机化学, 2010, 30(12): 1890-1897. |
| [4] | 曹鸿志,李祖义. 生物催化在非天然寡糖合成中的应用[J]. 有机化学, 2002, 22(1): 11-21. |
| [5] | 秦致辉,蔡孟深,李中军. 糖基亚苄基NaBH3CN-HCl选择性开环过程中乙酰基迁移 现象的研究[J]. 有机化学, 2001, 21(3): 214-217. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||