有机化学 ›› 2025, Vol. 45 ›› Issue (4): 1047-1096.DOI: 10.6023/cjoc202408019 上一篇 下一篇
综述与进展
收稿日期:2024-08-15
修回日期:2024-09-20
发布日期:2024-11-08
基金资助:
Yulan Fan, Xiaoying Zou, Xiaoqing Zhu, Lüyin Zheng( ), Wei Guo(
), Wei Guo( )
)
Received:2024-08-15
Revised:2024-09-20
Published:2024-11-08
Contact:
* E-mail: Supported by:文章分享
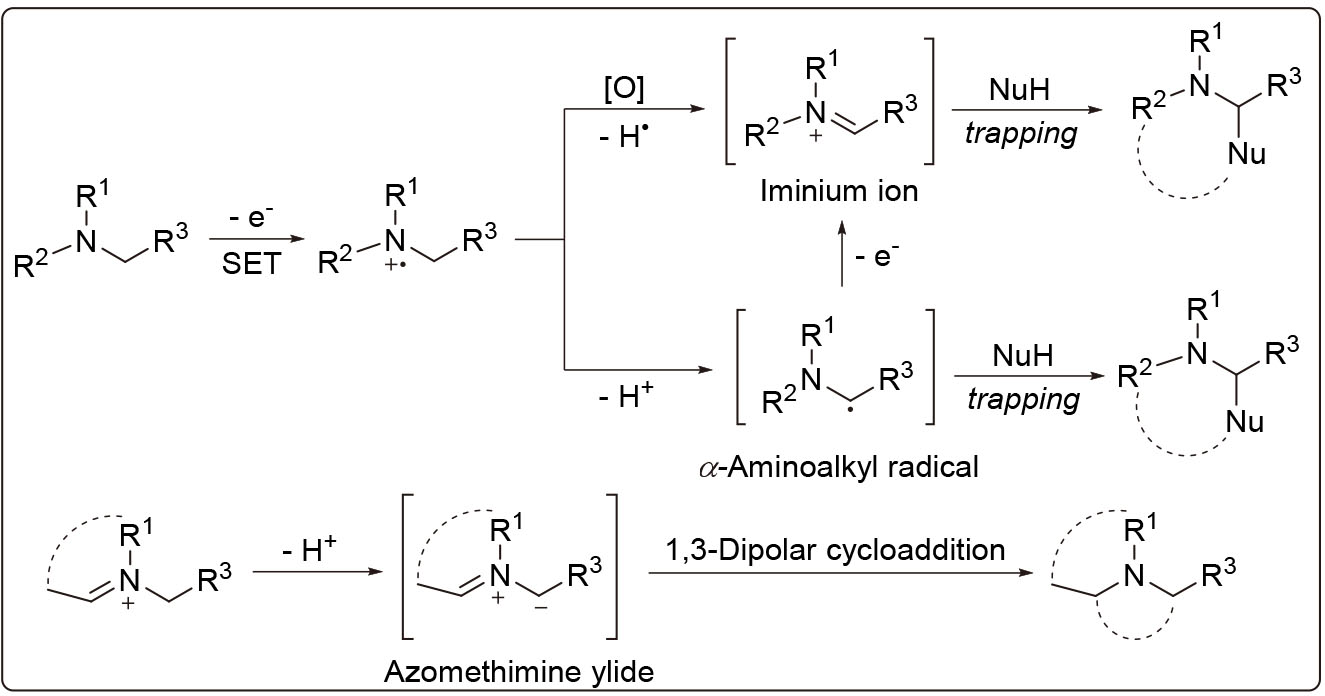
C(sp3)—H键官能团化是当前有机合成领域的研究热点. N-邻位C(sp3)—H键中的氮原子上孤对电子与邻位碳自由基的单占分子轨道(SOMO)中的电子发生离域后, 降低了C(sp3)—H键的解离能, 有利于引入各种亲核试剂或亲偶极试剂, 通过分子间或分子内亲核环化或偶极环化反应得到结构多样的含氮杂环化合物. 按照N-邻位C(sp3)—H键在不同条件下形成亚胺离子、α-氨基烷基自由基和甲亚胺叶立德等三种中间体进行分类, 综述了近年来N-邻位C(sp3)—H键官能团化合成含氮杂环化合物的研究进展, 阐述了可能经历的化学反应历程, 对其应用前景进行了展望.
范玉兰, 邹小颖, 朱小青, 郑绿茵, 郭维. N-邻位C(sp3)—H键官能团化合成含氮杂环化合物研究进展[J]. 有机化学, 2025, 45(4): 1047-1096.
Yulan Fan, Xiaoying Zou, Xiaoqing Zhu, Lüyin Zheng, Wei Guo. Progress in N-α-C(sp3)—H Bond Functionalization for the Synthesis of N-Heterocycles[J]. Chinese Journal of Organic Chemistry, 2025, 45(4): 1047-1096.
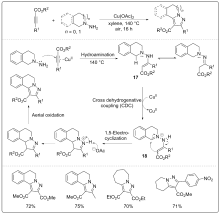

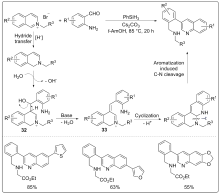
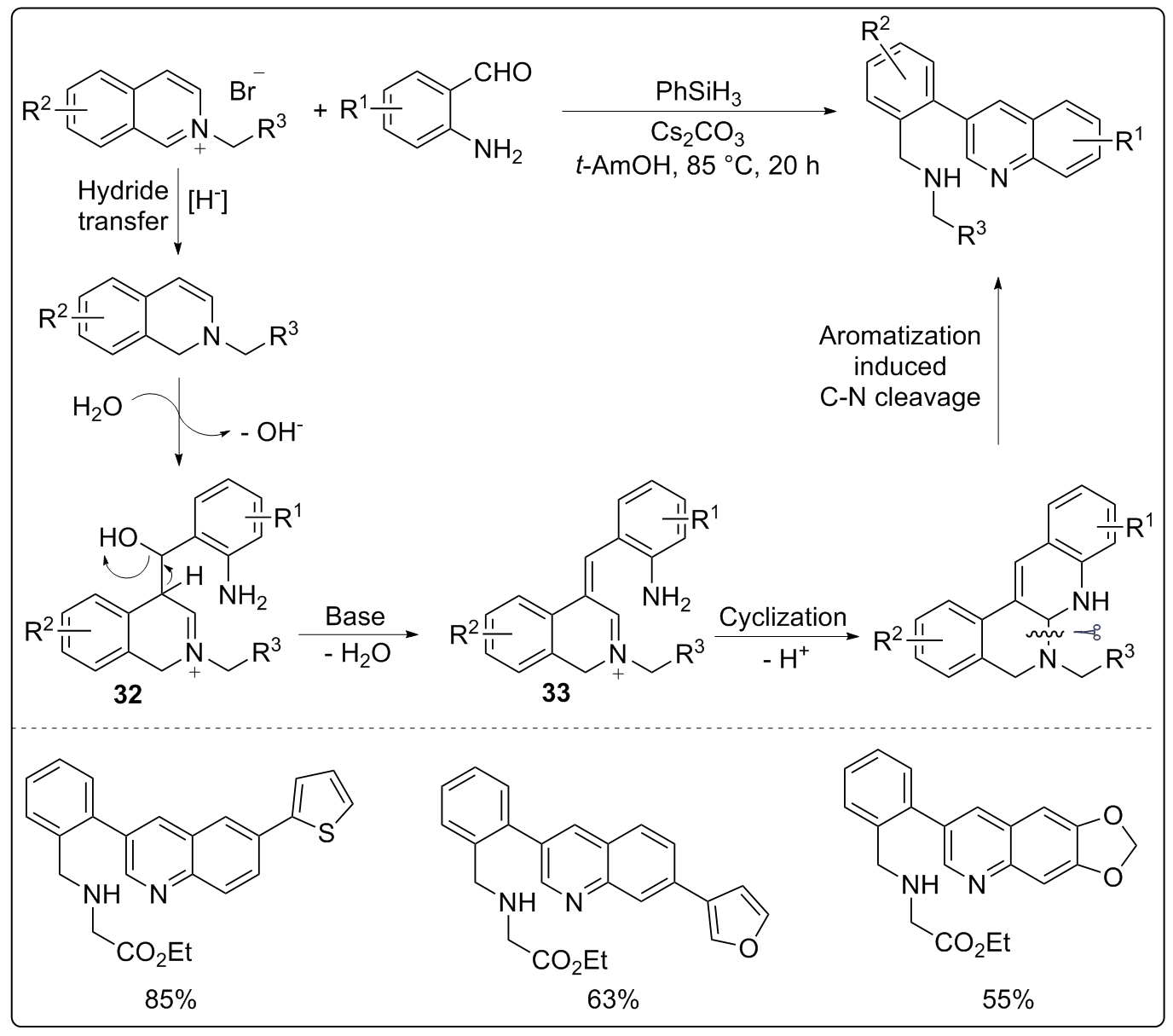

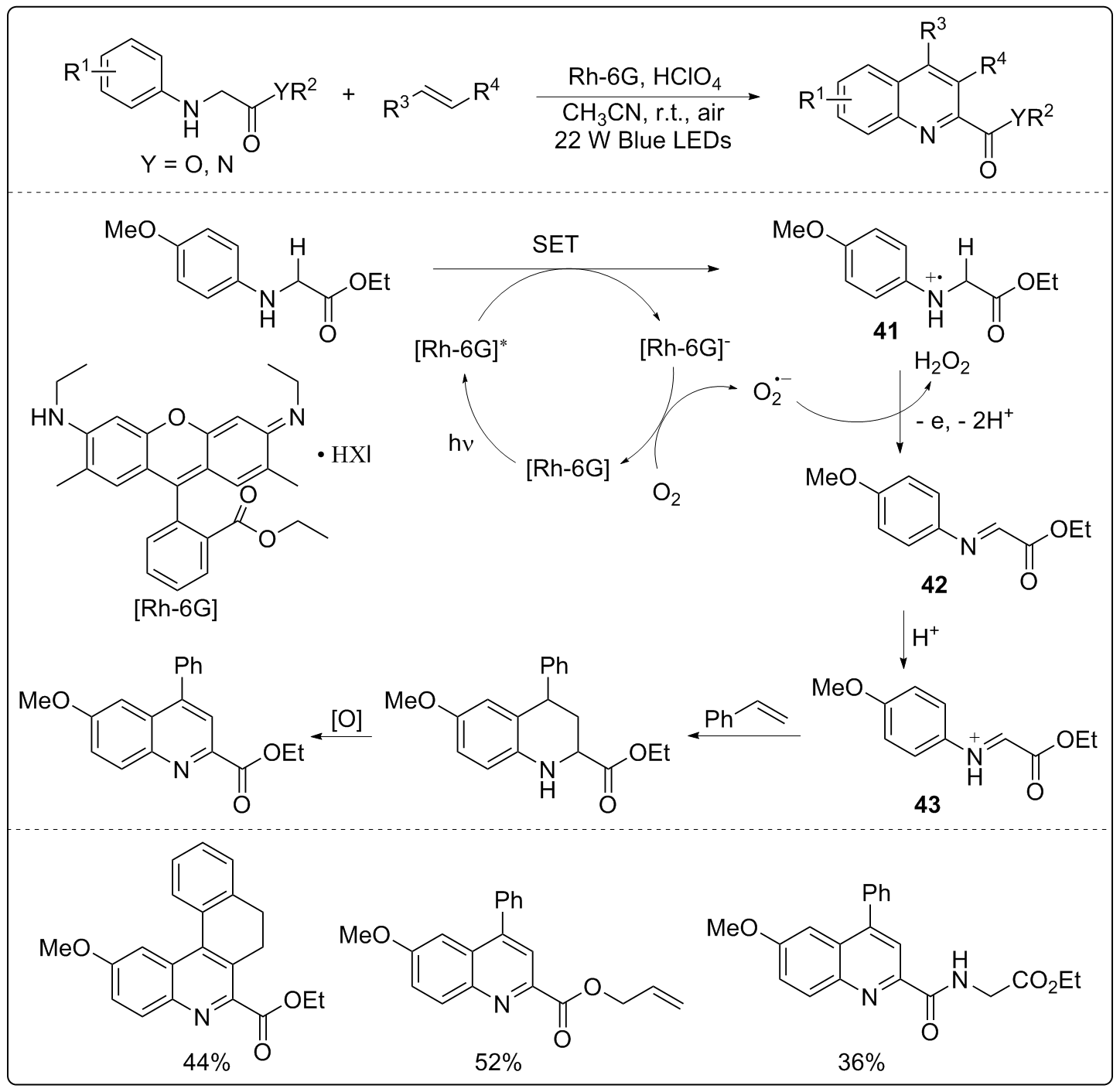
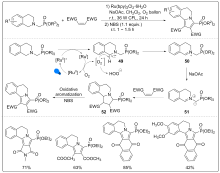

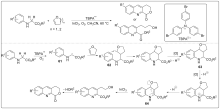

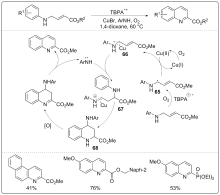
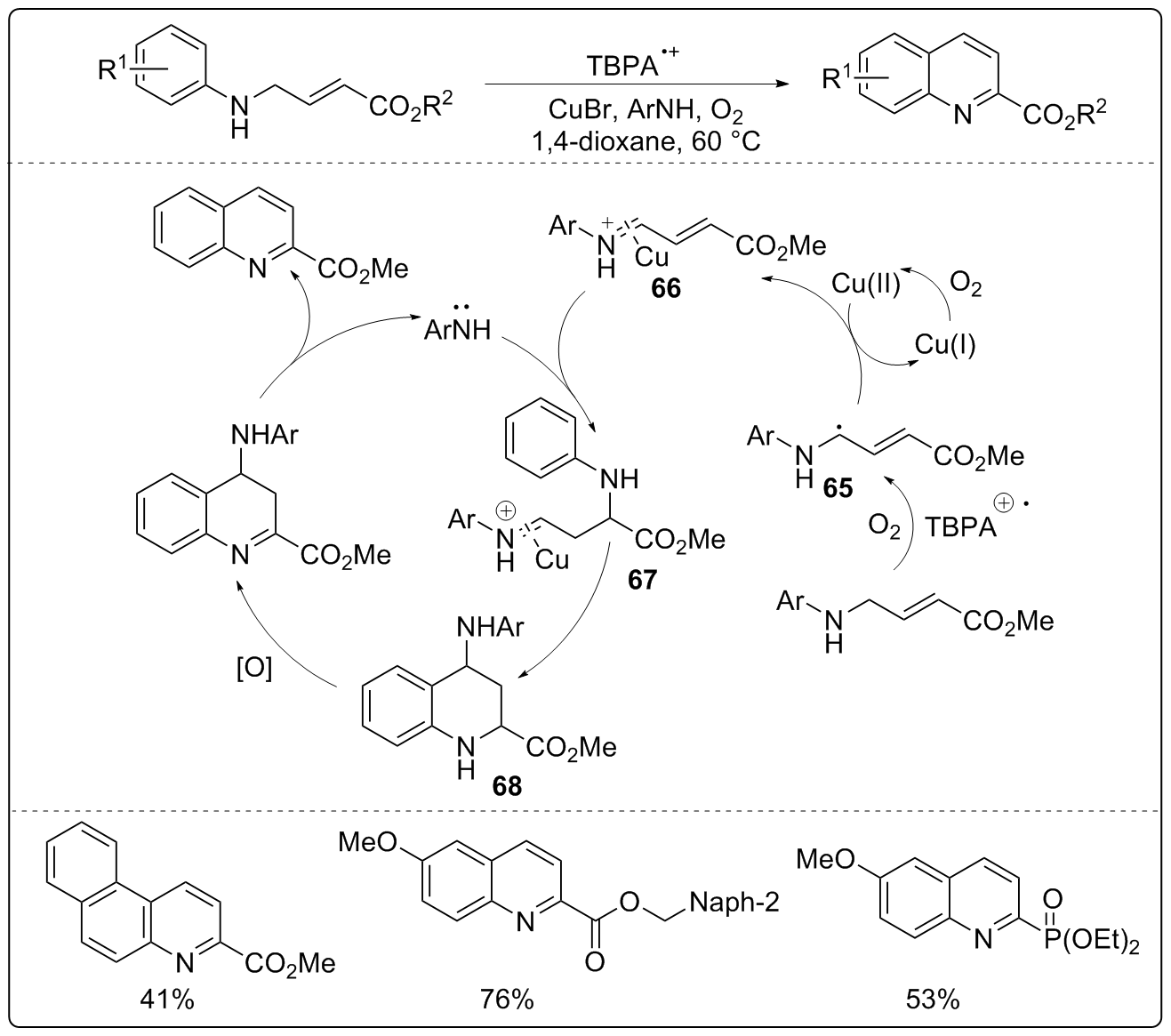
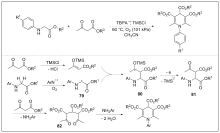

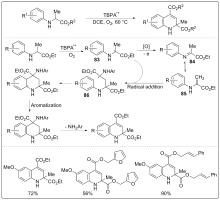

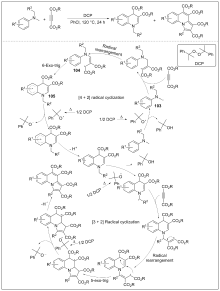

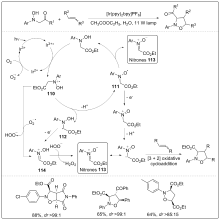

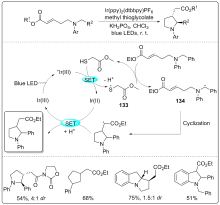

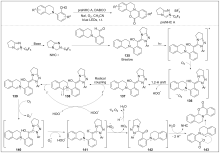

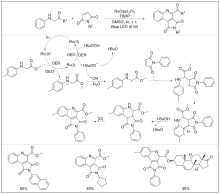
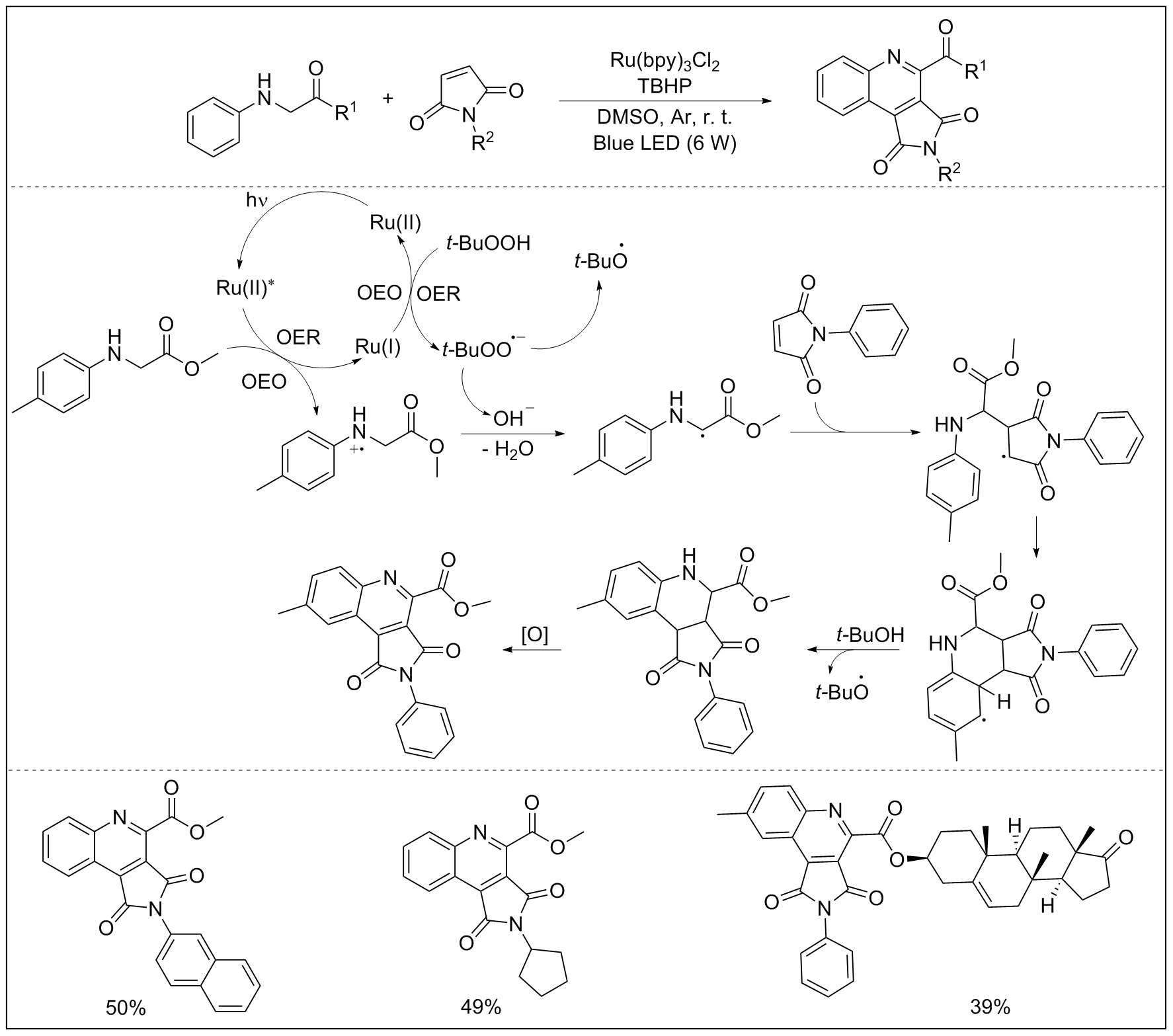
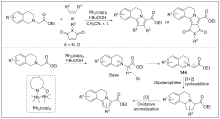
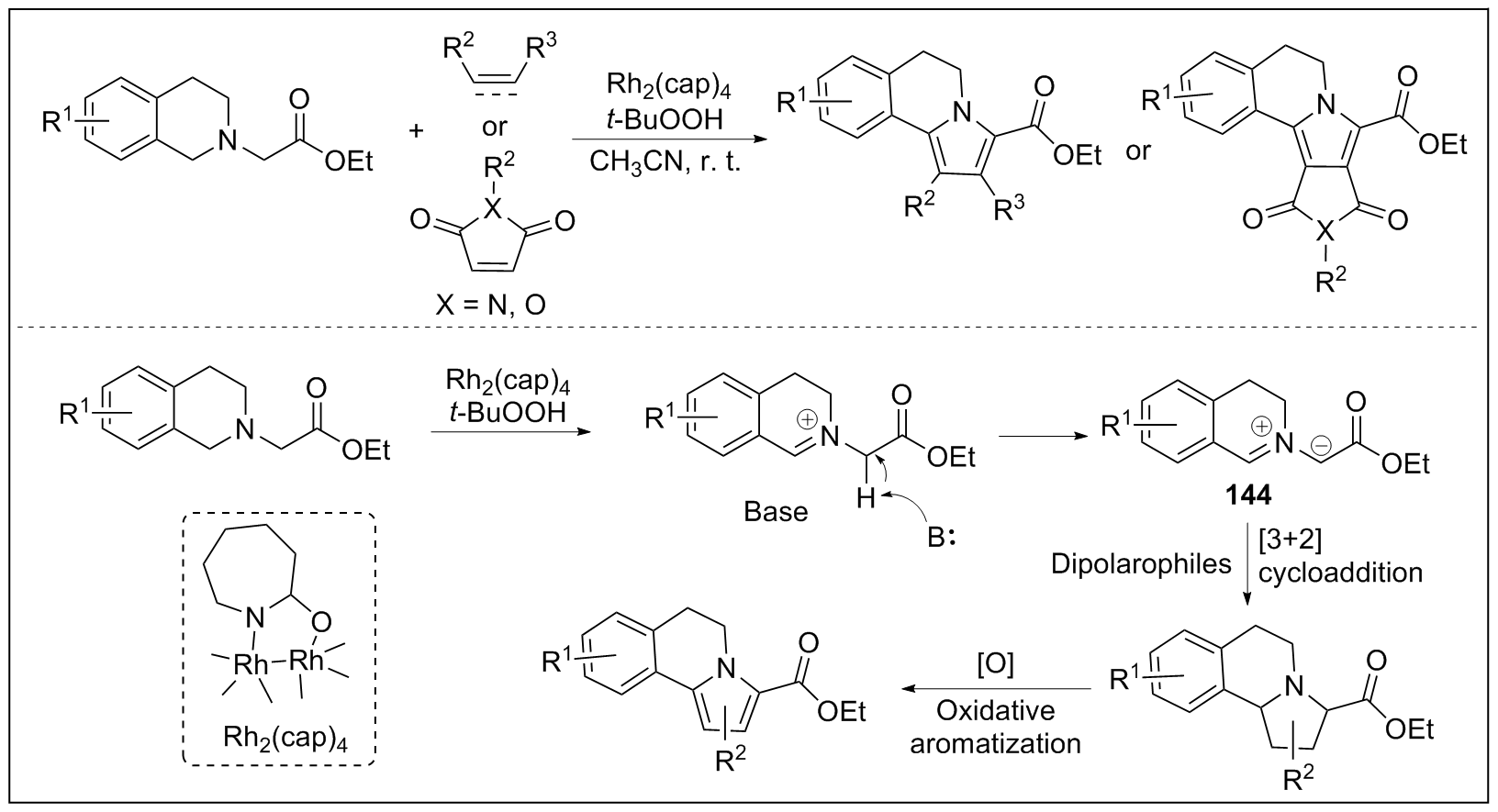
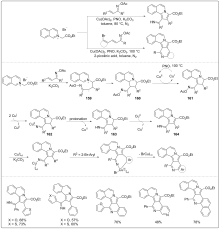

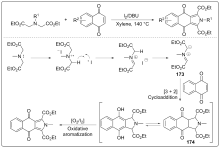
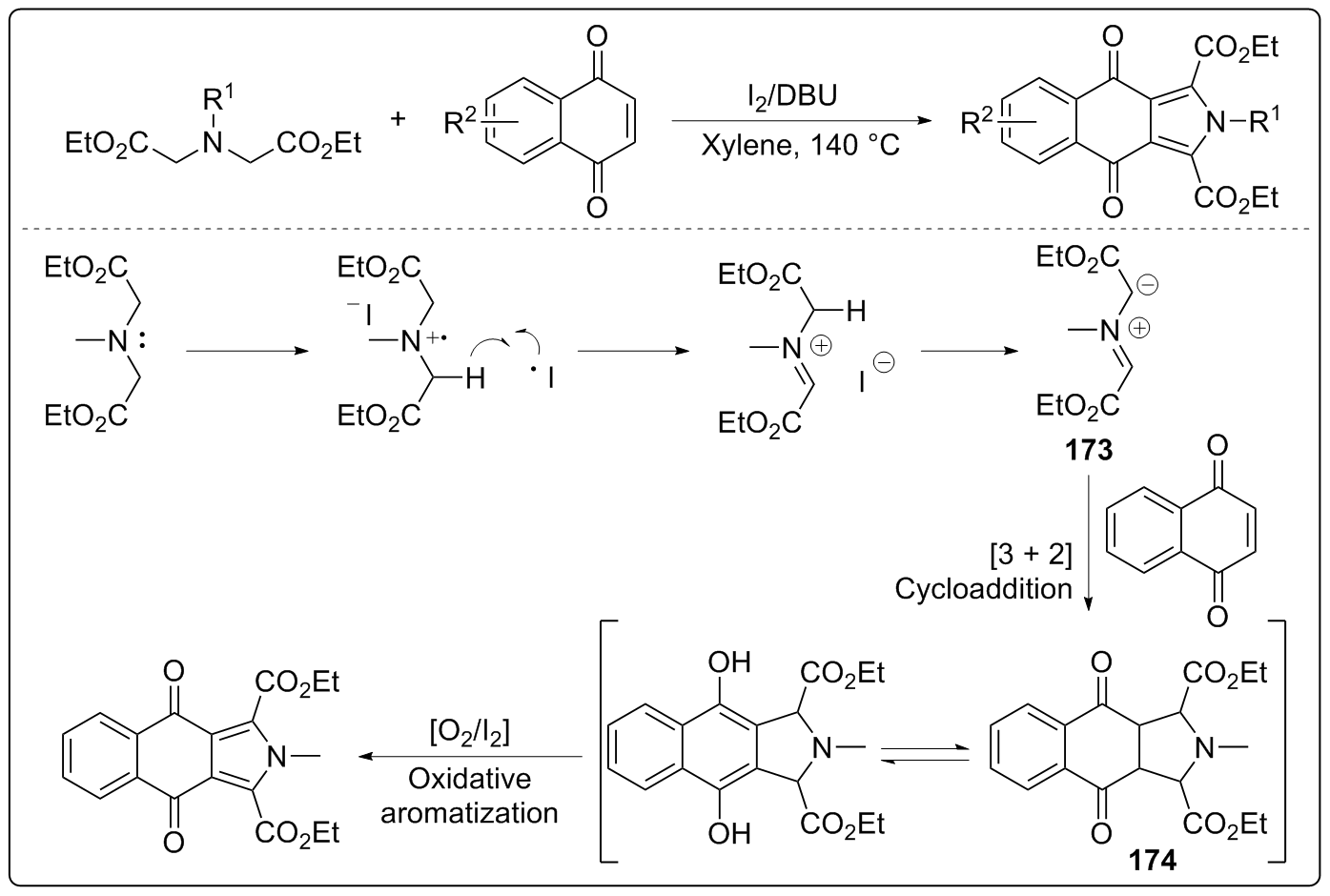
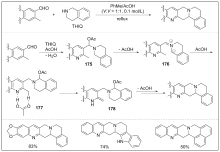

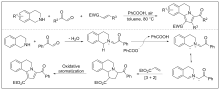
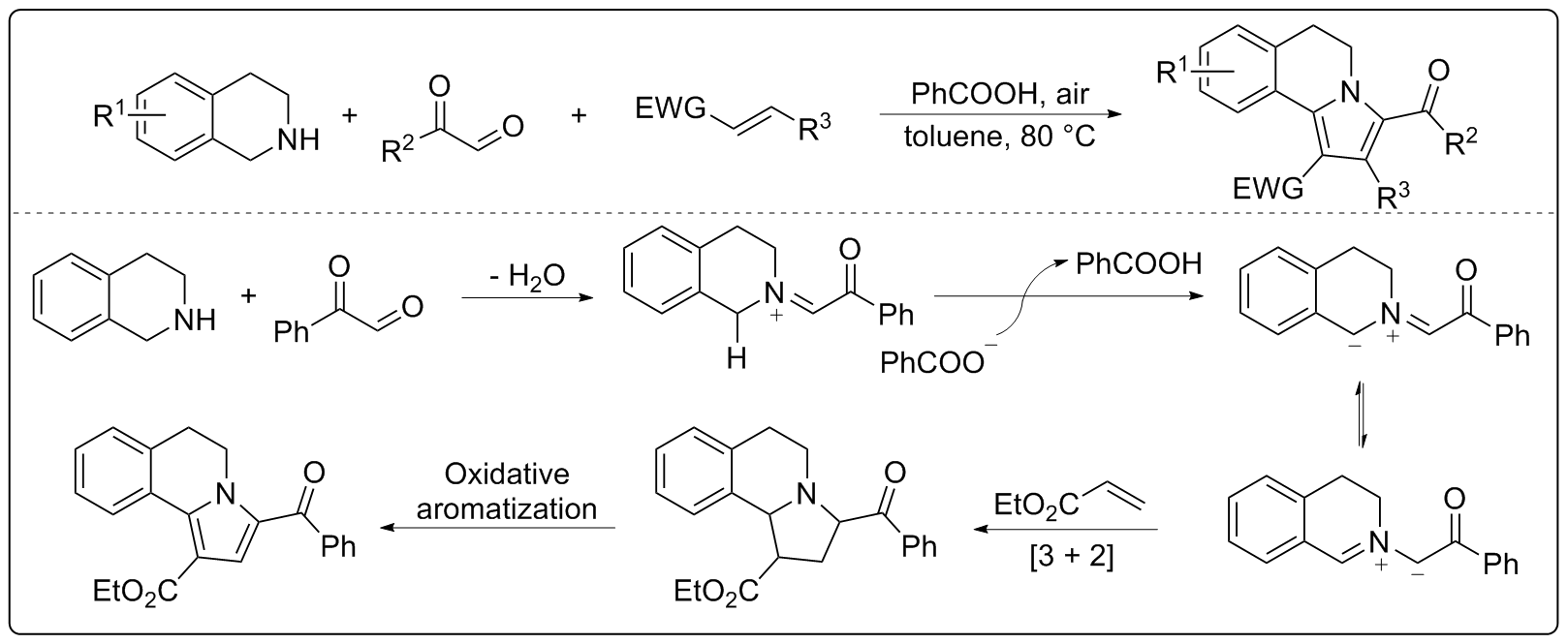
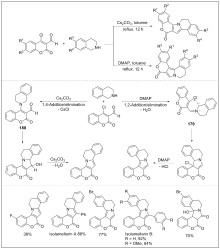

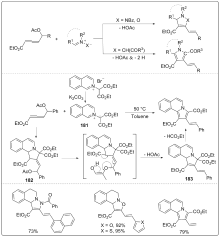

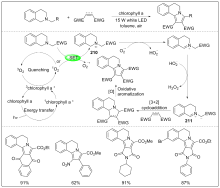

| [1] |
(a) Chugh, A.; Kumar, A.; Verma, A.; Kumar, S.; Kumar, P. Med. Chem. Res. 2020, 29, 1723.
|
|
(b) Kumari, S.; Maddeboina, K.; Bachu, R. D.; Boddu, S. H. S.; Trippier, P. C.; Tiwari, A. K. Drug Discovery Today 2022, 27, 103322.
|
|
|
(c) Mermer, A.; Keles, T.; Sirin, Y. Bioorg. Chem. 2021, 114, 105076.
|
|
| [2] |
(a) Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257.
doi: 10.1021/jm501100b pmid: 35557843 |
|
(b) Heravi, M. M.; Zadsirjan, V. RSC Adv. 2020, 10, 44247.
doi: 10.1039/d0ra09198g pmid: 35557843 |
|
| [3] |
(a) Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dincǎ, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; Kalmutzki, M.; Lächelt, U.; Ploetz, E.; Diercks, C. S.; Wuttke, S. Angew. Chem. Int. Ed. 2021, 60, 23975.
doi: 10.1002/anie.202106259 pmid: 33989445 |
|
(b) Zhang, Q.; Jiang, S.; Lv, T.; Peng, Y.; Pang, H. Adv. Mater. 2023, 35, 2305532.
pmid: 33989445 |
|
|
(c) Zheng, L.; Deng, L.; Zhong, Y.; Wang, Y.; Guo, W.; Fan, X. Parasitol. Res. 2021, 120, 3355.
pmid: 33989445 |
|
| [4] |
(a) Zheng, L.; Tao, K.; Guo, W. Adv. Synth. Catal. 2021, 363, 62.
|
|
(b) Guo, W.; Zhao, M.; Tan, W.; Zheng, L.; Tao, K.; Fan, X. Org. Chem. Front. 2019, 6, 2120.
|
|
| [5] |
(a) Godula, K.; Sames, D. Science 2006, 312, 67.
pmid: 16601184 |
|
(b) Bergman, R. G. Nature 2007, 446, 391.
pmid: 16601184 |
|
|
(c) Li, J.-Z.; Mei, L.; Yu, X.-C.; Wang, L.-T.; Cai, X.-E.; Li, T.; Wei, W.-T. Org. Chem. Front. 2022, 9, 5726.
pmid: 16601184 |
|
| [6] |
Zhu, Z.; Xiao, L.; Xie, Z.; Le, Z. Chin. J. Org. Chem. 2019, 39, 2345 (in Chinese).
|
|
(祝志强, 肖利金, 谢宗波, 乐长高, 有机化学, 2019, 39, 2345.)
doi: 10.6023/cjoc201903006 |
|
| [7] |
Luo, Y. R. Handbook of Bond Dissociation Energy in Organic Compound, CRC Press, Boca Raton, 2002.
|
| [8] |
(a) Hou, W.; Jia, X. Chin. J. Org. Chem. 2018, 38, 999 (in Chinese).
|
|
(侯文涛, 贾晓东, 有机化学, 2018, 38, 999.)
doi: 10.6023/cjoc201711037 |
|
|
(b) Liu, S.; Zhao, Z.; Wang, Y. Chem.-Eur. J. 2019, 25, 2423.
|
|
|
(c) Tashrifi, Z.; Khanaposhtani, M. M.; Larijani, B.; Mahdavi, M. Asian J. Org. Chem. 2021, 10, 2421.
doi: 10.1002/ajoc.202100407 |
|
| [9] |
(a) Hu, J.; Wang, J.; Nguyen, T. H.; Zheng, N. Beilstein J. Org. Chem. 2013, 9, 1977.
|
|
(b) Hoffmann, N. J. Phys. Org. Chem. 2015, 28, 121.
|
|
|
(c) Bao, X.; Jiang, W.; Liang, J.; Huo, C. Org. Chem. Front. 2020, 7, 2107.
|
|
|
(d) Kratena, N.; Marinic, B.; Donohoe, T. J. Chem. Sci. 2022, 13, 14213.
|
|
| [10] |
(a) Wolan, A.; Kowalska-Six, J. A.; Rajerison, H.; Césario, M.; Cordier, M.; Six, Y. Tetrahedron 2018, 74, 5248.
|
|
(b) Cui, H.-L. Org. Biomol. Chem. 2022, 20, 2779.
|
|
|
(c) Jacobs, J.; Van Hende, E.; Claessens, S.; De Kimpe, N. Curr. Org. Chem. 2011, 15, 1340.
|
|
| [11] |
Liu, P.; Li, Y.; Wang, H.; Wang, Z.; Hu, X. Tetrahedron Lett. 2012, 53, 6654.
|
| [12] |
Huo, C.; Yuan, Y.; Chen, F.; Tang, J.; Wang, Y. Org. Lett. 2015, 17, 4208.
|
| [13] |
Sengoden, M.; Bhowmick, A.; Punniyamurthy, T. Org. Lett. 2017, 19, 158.
doi: 10.1021/acs.orglett.6b03458 pmid: 27977211 |
| [14] |
Li, H.; Huang, S.; Wang, Y.; Huo, C. Org. Lett. 2018, 20, 92.
|
| [15] |
Basavaiah, D.; Lingaiah, B.; Reddy, G. C.; Sahu, B. C. Eur. J. Org. Chem. 2016, 2016, 2398.
|
| [16] |
Wang, W.; Sun, J.; Hu, H.; Liu, Y. Org. Biomol. Chem. 2018, 16, 1651.
|
| [17] |
Regalla, V. R.; Addada, R. R.; Chatterjee, A. Tetrahedron Lett. 2018, 59, 4161.
|
| [18] |
Xie, Z.; Li, F.; Niu, L.; Li, H.; Zheng, J.; Han, R.; Ju, Z.; Li, S.; Li, D. Org. Biomol. Chem. 2020, 18, 6889.
|
| [19] |
Huo, C.; Yuan, Y.; Wu, M.; Jia, X.; Wang, X.; Chen, F.; Tang, J. Angew. Chem. Int. Ed. 2014, 53, 13544.
|
| [20] |
Huang, S.; Bao, X.; Fu, Y.; Zhang, Y.; Quan, Z.; Huo, C. Asian J. Org. Chem. 2020, 9, 925.
|
| [21] |
Deb, M. L.; Borpatra, P. J.; Baruah, P. K. Green Chem. 2019, 21, 69.
|
| [22] |
Karjee, P.; Sarkar, T.; Kar, S.; Punniyamurthy, T. J. Org. Chem. 2020, 85, 8261.
|
| [23] |
Mao, W.; Zhao, H.; Zhang, M. Chem. Commun. 2022, 58, 4380.
|
| [24] |
Hu, B.; Dong, W.; Feng, Z.; Gao, X.; Gao, H.; Xie, X.; Zhang, Z. Asian J. Org. Chem. 2016, 5, 1467-1470.
|
| [25] |
Zhang, Y.; Li, S.; Zhu, Y.; Yang, X.; Zhou, H.; Li, Y. J. Org. Chem. 2020, 85, 6261.
doi: 10.1021/acs.joc.9b01440 pmid: 32281374 |
| [26] |
Wang, J.; Li, L.; Guo, Y.; Li, S.; Wang, S.; Li, Y.; Zhang, Y. Org. Biomol. Chem. 2020, 18, 8179.
|
| [27] |
Luo, K.; Zhao, Y.; Tang, Z.; Li, W.; Lin, J.; Jin, Y. Org. Lett. 2022, 24, 6335.
|
| [28] |
Borpatra, P. J.; Deb, M. L.; Baruah, P. K. Tetrahedron Lett. 2017, 58, 4006.
|
| [29] |
Wang, L.; Ma, T.; Qiao, M.; Wu, Q.; Shi, D.; Xiao, W. Synthesis 2019, 51, 522.
|
| [30] |
Vijay, M.; Kumar, S. V.; Satheesh, V.; Ananthappan, P.; Srivastava, H. K.; Ellairaja, S.; Vasantha, V. S.; Punniyamurthy, T. Org. Lett. 2019, 21, 7649.
|
| [31] |
Yu, D.; Liu, Y.; Che, C.-M. Org. Chem. Front. 2022, 9, 2779.
|
| [32] |
Sakai, N.; Matsumoto, S.; Ogiwara, Y. Tetrahedron Lett. 2016, 57, 5449.
|
| [33] |
Ma, X.; Zhu, Y.; Lü, S.; Zhang, L.; Luo, L.; Jia, X. Tetrahedron Lett. 2016, 57, 1528.
|
| [34] |
Jia, X.; Li, P.; Shao, Y.; Yuan, Y.; Hou, W; Liu, X.; Zhang, X.; Ji, H. Chem.-Asian J. 2017, 12, 1719.
|
| [35] |
Zhang, X.; Li, P.; Yuan, Y.; Jia, X. Chin. J. Org. Chem. 2018, 38, 2435 (in Chinese).
|
|
(张学文, 李鹏飞, 袁宇, 贾晓东, 有机化学, 2018, 38, 2435.)
doi: 10.6023/cjoc201804012 |
|
| [36] |
Wei, W.-T.; Teng, F.; Li, Y.; Song, R.-J.; Li, J.-H. Org. Lett. 2019, 21, 6285.
|
| [37] |
Hwang, J. Y.; Ji, A. Y.; Lee, S. H.; Kang, E. J. Org. Lett. 2020, 22, 16.
|
| [38] |
Yuan, Y.; Zhang, S.; Sun, Z.; Su, Y.; Ma, Q.; Yuan, Y.; Jia, X. Org. Lett. 2020, 22, 6294.
doi: 10.1021/acs.orglett.0c02054 pmid: 32806190 |
| [39] |
Jia, X.; Wang, Y.; Peng, F.; Huo, C.; Yu, L.; Liu, J.; Wang, X. Adv. Synth. Catal. 2014, 356, 1210.
|
| [40] |
Lü, S.; Zhu, Y.; Ma, X.; Jia, X. Adv. Synth. Catal. 2016, 358, 1004.
|
| [41] |
Jia, X.; Zhu, Y.; Yuan, Y.; Zhang, X.; Lü, S.; Zhang, L.; Luo, L. ACS Catal. 2016, 6, 6033.
|
| [42] |
Luo, L.; Zhao, X.; Zhang, L.; Yuan, Y.; Lü, S.; Jia, X. Tetrahedron Lett. 2016, 57, 5830.
|
| [43] |
Jia, X.; Lü, S.; Yuan, Y.; Zhang, X.; Zhang, L.; Luo, L. Org. Biomol. Chem. 2017, 15, 2931.
|
| [44] |
Ji, H.; Zhu, Y.; Shao, Y.; Liu, J.; Yuan, Y.; Jia, X. J. Org. Chem. 2017, 82, 9859.
|
| [45] |
Luo, Z.; Han, X.; Liu, C.; Liu, Q.; Li, R.; Liu, P.; Xu, X. Synthesis 2020, 52, 1067.
|
| [46] |
Nakajima, K.; Kitagawa, M.; Ashida, Y.; Miyake, Y.; Nishibayashi, Y. Chem. Commun. 2014, 50, 8900.
|
| [47] |
Hou, H.; Zhu, S.; Pan, F.; Rueping, M. Org. Lett. 2014, 16, 2872.
|
| [48] |
Liang, Z.; Xu, S.; Tian, W.; Zhang, R. Beilstein J. Org. Chem. 2015, 11, 425.
|
| [49] |
Hsu, C.-W.; Sundén, H. Org. Lett. 2018, 20, 2051.
|
| [50] |
Yang, X.-L.; Guo, J.-D.; Lei, T.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Org. Lett. 2018, 20, 2916.
|
| [51] |
Yuan, X.; Wu, X.; Dong, S.; Wu, G.; Ye, J. Org. Biomol. Chem. 2016, 14, 7447.
|
| [52] |
Zhao, Y.; Chen, J.-R.; Xiao, W.-J. Org. Lett. 2016, 18, 6304.
pmid: 27978702 |
| [53] |
Liu, J.; Xie, J.; Zhu, C. Org. Chem. Front. 2017, 4, 2433.
|
| [54] |
Natarajan, P.; Chuskit, D.; Priya Green Chem. 2019, 21, 4406.
|
| [55] |
Gao, Z.-H.; Xia, Z.-H.; Dai, L.; Ye, S. Adv. Synth. Catal. 2020, 362, 1819.
|
| [56] |
Zhang, Y.; Jiang, W.; Bao, X.; Qiu, Y.; Yuan, Y.; Yang, C.; Huo, C. Chin. J. Chem. 2021, 39, 3238.
|
| [57] |
Wang, H.-T.; Lu, C.-D. Tetrahedron Lett. 2013, 54, 3015.
|
| [58] |
Chen, X.-H.; Pan, Y.-Y.; Wang, W.-X.; Cui, H.-L. Synlett 2022, 33, 1645.
|
| [59] |
Sugimoto, K.; Yamamoto, N.; Tominaga, D.; Matsuya, Y. Org. Lett. 2015, 17, 1320.
doi: 10.1021/acs.orglett.5b00320 pmid: 25700009 |
| [60] |
Motornov, V. A.; Tabolin, A. A.; Nelyubina, Y. V.; Nenajdenko, V, G.; Ioffe, S. L. Org. Biomol. Chem. 2019, 17, 1442.
|
| [61] |
Motornov, V. A.; Tabolin, A. A.; Ioffe, S. L. New J. Chem. 2022, 46, 4134.
|
| [62] |
Miao, C.-B.; Qiang, X.-Q.; Xu, X.; Song, X.-Q.; Zhou, S.-Q.; Lyu, X.; Yang, H.-T. Org. Lett. 2022, 24, 3828.
|
| [63] |
Huang, H.-M.; Li, Y.-J.; Ye, Q.; Yu, W.-B.; Han, L.; Jia, J.-H.; Gao, J.-R. J. Org. Chem. 2014, 79, 1084.
doi: 10.1021/jo402540j pmid: 24393026 |
| [64] |
Huang, H.-M.; Huang, F.; Li, Y.-J.; Jia, J.-H.; Ye, Q.; Han, L.; Gao, J.-R. RSC Adv. 2014, 4, 27250.
|
| [65] |
Yang, Z.; Lu, N.; Wei, Z.; Cao, J.; Liang, D.; Duan, H.; Lin, Y. J. Org. Chem. 2016, 81, 11950.
|
| [66] |
Huang, H.-M.; Gao, J.-R.; Ye, Q.; Yu, W.-B.; Sheng, W.-J.; Li, Y.-J. RSC Adv. 2014, 4, 15526.
|
| [67] |
Zhu, Z.; Seidel, D. Org. Lett. 2017, 19, 2841.
|
| [68] |
Zheng, K.; Zhuang, S.; You, M.; Shu, W.; Wu, A.; Wu, Y. ChemistrySelect 2017, 2, 10762.
|
| [69] |
Zheng, K.-L.; You, M.-Q.; Shu, W.-M.; Wu, Y.-D.; Wu, A.-X. Org. Lett. 2017, 19, 2262.
|
| [70] |
Vyasamudri, S.; Yang, D.-Y. J. Org. Chem. 2019, 84, 3662.
doi: 10.1021/acs.joc.8b03259 pmid: 30807162 |
| [71] |
Wang, K.-K.; Li, Y.-L.; Wang, Z.-Y.; Ma, X.; Mei, Y.-L.; Zhang, S.-S.; Chen, R. J. Heterocycl. Chem. 2020, 57, 1456.
|
| [72] |
Li, F.; Chen, J.; Hou, Y.; Li, Y.; Wu, X.-Y.; Tong, X. Org. Lett. 2015, 17, 5376.
|
| [73] |
Jadala, C.; Reddy, V. G.; Krishna, N. H.; Shankaraiah, N.; Kamal, A. Org. Biomol. Chem. 2020, 18, 8694.
|
| [74] |
Hou, X.; Zhou, S.; Li, Y.; Guo, M.; Zhao, W.; Tang, X.; Wang, G. Org. Lett. 2020, 22, 9313.
|
| [75] |
Aksenov, A. V.; Arutiunov, N. A.; Kirilov, N. K.; Aksenov, D. A.; Grishin, I. Y.; Aksenov, N. A.; Wang, H.; Du, L.; Betancourt, T.; Pelly, S. C.; Kornienko, A.; Rubin, M. Org. Biomol. Chem. 2021, 19, 7234.
|
| [76] |
Yang, L.-M.; Zhang, Y.-Y.; Deng, J.-T.; Ma, A.-J.; Zhang, X.-Z.; Zhang, S.-Y.; Peng, J.-B. Asian J. Org. Chem. 2021, 10, 1679.
|
| [77] |
Izmest'ev, A. N.; Motornov, V. A.; Vinogradov, D. B.; Ioffe, S. L.; Kravchenko, A. N.; Gazieva, G. A. Org. Chem. Front. 2022, 9, 4998.
|
| [78] |
Zhang, X.; Wang, X.; Jiao, C.; Zhao, J.; Liu, X.; Zhang, G. Org. Chem. Front. 2022, 9, 5516.
|
| [79] |
Wang, Q.; Yuan, T.; Liu, Q.; Xu, Y.; Xie, G.; Lv, X.; Ding, S.; Wang, X.; Li, C. Chem. Commun. 2019, 55, 8398.
|
| [80] |
Kumar, R.; Banerjee, P. J. Org. Chem. 2021, 86, 16104.
|
| [81] |
Koohgard, M.; Hosseini-Sarvari, M. J. Photochem. Photobiol. A 2021, 404, 112877.
|
| [82] |
Zheng, L.; Zou, X.; Yang, X.; Deng, L.; Guo, W. Adv. Synth. Catal. 2023, 365, 3629.
|
| [83] |
Mailloux, M. J.; Fleming, G. S.; Kumta, S. S.; Beeler, A. B. Org. Lett. 2021, 23, 525.
doi: 10.1021/acs.orglett.0c04050 pmid: 33395312 |
| [84] |
Qu, Z.; Zhang, F.; Deng, G.-J.; Huang, H. Org. Lett. 2019, 21, 8239.
|
| [85] |
Wang, D.; Xiao, F.; Zhang, F.; Huang, H.; Deng, G.-J. Chin. J. Chem. 2021, 39, 87.
|
| [86] |
Kadu, V. D.; Khadul, S. P.; Kothe, G. J.; Mali, G. A. Asian J. Org. Chem. 2022, 11, e202200162.
|
| [87] |
Camats, M.; Favier, I.; Mallet-Ladeira, S.; Pla, D.; Gómez, M. Org. Biomol. Chem. 2022, 20, 219.
|
| [88] |
Kadu, V. D.; Sankala, N. C.; Hublikar, M. G.; Bansode, S. I.; Bhosale, R. B. Synthesis 2024, 56, 2277.
|
| [89] |
Wang, K.; Sun, Y.; Li, B.; Zhang, X.; Fan, X. Org. Lett. 2024, 26, 3091.
|
| [90] |
Shen, J.; Cai, D.; Kuai, C.; Liu, Y.; Wei, M.; Cheng, G.; Cui, X. J. Org. Chem. 2015, 80, 6584.
|
| [91] |
Qin, Q.; Yu, S. Org. Lett. 2015, 17, 1894.
|
| [1] | 洪科苗, 黄晶晶, 姚铭瀚, 徐新芳. 氮宾/炔烃复分解串联反应研究进展[J]. 有机化学, 2022, 42(2): 344-352. |
| [2] | 周淑蕊, 温凯歌, 曾兴平. 亚胺及其类似物的催化不对称炔基化反应新进展[J]. 有机化学, 2021, 41(2): 471-489. |
| [3] | 赵赫, 程冬萍, 许孝良. α-氨基烷基自由基在可见光催化中的应用[J]. 有机化学, 2021, 41(2): 642-660. |
| [4] | 张金, 刘佳, 马养民, 程佩. 纳米金属氧化物催化含氮杂环类化合物的合成研究进展[J]. 有机化学, 2017, 37(3): 555-565. |
| [5] | 杨锦明, 褚雪强, 纪顺俊. 吡咯烷酮衍生物的直接胺化: 构建偕二胺衍生物[J]. 有机化学, 2014, 34(12): 2462-2470. |
| [6] | 李筱芳, 易荣琼, 刘彬, 李志奎, 于贤勇, 易平贵. 甲亚胺叶立德的1,3-偶极环加成反应合成螺吲哚里西啶类化合物[J]. 有机化学, 2012, 32(12): 2309-2314. |
| [7] | 李筱芳, 于贤勇, 冯亚青. 1,3-偶极环加成反应合成螺噻唑并[3,2-a]嘧啶类化合物[J]. 有机化学, 2010, 30(05): 735-739. |
| [8] | 罗培松, 汤日元, 钟平, 李金恒. 炔烃分子内环化反应合成含氮杂环化合物研究进展[J]. 有机化学, 2009, 29(12): 1924-1937. |
| [9] | 李筱芳a,b; 于贤勇a; 冯亚青*,b. 区域和立体选择性合成新型螺羟吲哚类化合物[J]. 有机化学, 2009, 29(07): 1129-1132. |
| [10] | 王进军,谢磊,康明芹,张敏,李付国,崔丙存. 氢化喹啉及其芳(杂)环稠合衍生物的合成[J]. 有机化学, 2005, 25(07): 830-834. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||