有机化学 ›› 2025, Vol. 45 ›› Issue (12): 4375-4383.DOI: 10.6023/cjoc202501004 上一篇 下一篇
研究论文
收稿日期:2025-04-10
修回日期:2025-05-25
发布日期:2025-08-18
通讯作者:
王敏
基金资助:
Ruijie Yang, Xin Wang, Zhihui Zhu, Yiming Xu, Zhiguo Song, Min Wang*( )
)
Received:2025-04-10
Revised:2025-05-25
Published:2025-08-18
Contact:
Min Wang
Supported by:文章分享

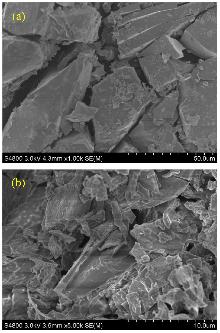
在溶剂热条件下, 以四水合硫酸锌(ZnSO4•4H2O)、4-羟基苯磺酸钠二水合物(C6H5NaO4S•2H2O)、1,6-二(1H-1,2,4-三氮唑)己烷(btx)为原料合成了一种新的锌配合物[Zn(btx)2(H2O)4]•(p-HOC6H4SO3)4. 通过X射线单晶衍射、红外光谱、X射线粉末衍射、氮气吸附-脱附测试、扫描电镜对其进行了表征. 考察了该配合物催化芳香醛与邻氨基苯甲酰胺合成2-取代-2,3-二氢喹唑啉-4(1H)-酮的性能. 实验结果表明, 使用少量的催化剂, 在短时间内可以得到较高的产率; 芳香醛苯环上取代基位置相同时, 取代基的吸电子能力越强, 产物产率越高. 运用Gaussian 16程序对16种不同结构的芳香醛分子分别进行优化计算, 计算结果可得, 当取代基位置相同时, 取代基的吸电子能力越强, 芳香醛的最低未占据分子轨道(LUMO)能量越低, 反应活性越强, 有助于反应的顺利进行, 与催化实验的结论相吻合. 最后采用密度泛函理论推测出锌配合物的活性位点, 阐述了其作为催化剂催化合成2-取代-2,3-二氢喹唑啉-4(1H)-酮可能的反应机理.
杨瑞杰, 王鑫, 朱志慧, 徐一铭, 宋志国, 王敏. 一种新型Zn(II)配合物绿色催化合成2-取代-2,3-二氢喹唑啉-4(1H)-酮[J]. 有机化学, 2025, 45(12): 4375-4383.
Ruijie Yang, Xin Wang, Zhihui Zhu, Yiming Xu, Zhiguo Song, Min Wang. Green Synthesis of 2-Substituted-2,3-dihydroquinazolin-4(1H)-ones Catalyzed by a Novel Zn(II) Complex[J]. Chinese Journal of Organic Chemistry, 2025, 45(12): 4375-4383.
| Parameter | Value |
|---|---|
| Formula | C44H68ZnN12O24S4 |
| Formula weight | 1314.5 |
| Crystal system | Triclinic |
| Space group | P-1 |
| a/nm | 0.75461(3) |
| b/nm | 0.91215(3) |
| c/nm | 1.24011(10) |
| α/(°) | 75.1310(10) |
| β/(°) | 89.0470(10) |
| γ/(°) | 78.3130(10) |
| V/nm3 | 0.80734(5) |
| Z | 1 |
| Dc/(mg•m-3) | 1.522 |
| Goodness-of-fit F2 | 1.100 |
| R1a, wR2b | 0.0497, 0.1492 |
| R1, wR2 (all data) | 0.0522, 0.1520 |
| Parameter | Value |
|---|---|
| Formula | C44H68ZnN12O24S4 |
| Formula weight | 1314.5 |
| Crystal system | Triclinic |
| Space group | P-1 |
| a/nm | 0.75461(3) |
| b/nm | 0.91215(3) |
| c/nm | 1.24011(10) |
| α/(°) | 75.1310(10) |
| β/(°) | 89.0470(10) |
| γ/(°) | 78.3130(10) |
| V/nm3 | 0.80734(5) |
| Z | 1 |
| Dc/(mg•m-3) | 1.522 |
| Goodness-of-fit F2 | 1.100 |
| R1a, wR2b | 0.0497, 0.1492 |
| R1, wR2 (all data) | 0.0522, 0.1520 |
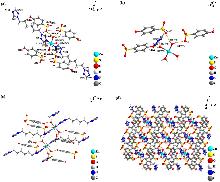

| Bond | Length/nm | Bond angle | Angle/(°) |
|---|---|---|---|
| Zn(1)—N(1) | 0.2101(3) | O(2W)—Zn(1)—O(1W) | 88.82(11) |
| Zn(1)—N(1)#1 | 0.2101(3) | O(2W)#1—Zn(1)—O(1W) | 91.18(11) |
| Zn(1)—O(1W) | 0.2142(3) | N(1)#1—Zn(1)—O(2W)#1 | 88.63(10) |
| Zn(1)—O(1W)#1 | 0.2142(3) | N(1)#1—Zn(1)—O(1W)#1 | 91.23(11) |
| Zn(1)—O(2W) | 0.2105(2) | O(2W)—Zn(1)—O(1W)#1 | 91.18(11) |
| Zn(1)—O(1W)#1 | 0.2105(2) | N(1)—Zn(1)—O(2W) | 88.63(10) |
| Bond | Length/nm | Bond angle | Angle/(°) |
|---|---|---|---|
| Zn(1)—N(1) | 0.2101(3) | O(2W)—Zn(1)—O(1W) | 88.82(11) |
| Zn(1)—N(1)#1 | 0.2101(3) | O(2W)#1—Zn(1)—O(1W) | 91.18(11) |
| Zn(1)—O(1W) | 0.2142(3) | N(1)#1—Zn(1)—O(2W)#1 | 88.63(10) |
| Zn(1)—O(1W)#1 | 0.2142(3) | N(1)#1—Zn(1)—O(1W)#1 | 91.23(11) |
| Zn(1)—O(2W) | 0.2105(2) | O(2W)—Zn(1)—O(1W)#1 | 91.18(11) |
| Zn(1)—O(1W)#1 | 0.2105(2) | N(1)—Zn(1)—O(2W) | 88.63(10) |
| D—H…A | Bond length (D—H)/nm | Bond length (H…A)/nm | Bond length (D…A)/nm | ∠DHA/(°) |
|---|---|---|---|---|
| O(1W)—H(1WA)…O(1) | 0.085 | 0.220 | 0.2793(4) | 150.9 |
| O(1W)—H(1WB)…O(1)#4 | 0.085 | 0.197 | 0.2809(4) | 167.0 |
| O(2W)—H(2WA)…O(4)#4 | 0.085 | 0.196 | 0.2769(4) | 158.9 |
| O(2W)—H(2WB)…N(3)#5 | 0.085 | 0.205 | 0.2870(4) | 161.7 |
| D—H…A | Bond length (D—H)/nm | Bond length (H…A)/nm | Bond length (D…A)/nm | ∠DHA/(°) |
|---|---|---|---|---|
| O(1W)—H(1WA)…O(1) | 0.085 | 0.220 | 0.2793(4) | 150.9 |
| O(1W)—H(1WB)…O(1)#4 | 0.085 | 0.197 | 0.2809(4) | 167.0 |
| O(2W)—H(2WA)…O(4)#4 | 0.085 | 0.196 | 0.2769(4) | 158.9 |
| O(2W)—H(2WB)…N(3)#5 | 0.085 | 0.205 | 0.2870(4) | 161.7 |

| Entry | R | LUMO/a.u. | Yield/% | m.p./℃ | |
|---|---|---|---|---|---|
| Found | Reported | ||||
| 1 | H | -0.06291 | 92.5, 91.7, 90.8b | 219~222 | 219~222[ |
| 2 | 2-NO2 | -0.10687 | 95.2 | 190~191 | 190~192[ |
| 3 | 2-Cl | -0.07927 | 93.3 | 203~205 | 202~20422] |
| 4 | 2-Br | -0.07660 | 91.8 | 179~181 | 179~182[ |
| 5 | 3-NO2 | -0.10429 | 94.7 | 201~203 | 200~203[ |
| 6 | 3-Cl | -0.07463 | 92.1 | 184~186 | 186~187[ |
| 7 | 3-Br | -0.07444 | 91.6 | 226~228 | 227~228[ |
| 8 | 3-CH3 | -0.06000 | 88.1 | 204~206 | 204[ |
| 9 | 3-CH3O | -0.05980 | 86.9 | 156~158 | 157~158[ |
| 10 | 3-OH | -0.05253 | 86.2 | 224~226 | 223~225[ |
| 11 | 4-NO2 | -0.11459 | 97.3 | 198~200 | 198~200[ |
| 12 | 4-Cl | -0.07305 | 91.7 | 199~202 | 198~201[ |
| 13 | 4-Br | -0.07019 | 90.8 | 194~197 | 195~197[ |
| 14 | 4-CH3 | -0.05901 | 86.7 | 232~234 | 233~234[ |
| 15 | 4-CH3O | -0.05166 | 85.9 | 189~191 | 188~190[ |
| 16 | 4-OH | -0.05108 | 84.5 | 184 | 182[ |
| Entry | R | LUMO/a.u. | Yield/% | m.p./℃ | |
|---|---|---|---|---|---|
| Found | Reported | ||||
| 1 | H | -0.06291 | 92.5, 91.7, 90.8b | 219~222 | 219~222[ |
| 2 | 2-NO2 | -0.10687 | 95.2 | 190~191 | 190~192[ |
| 3 | 2-Cl | -0.07927 | 93.3 | 203~205 | 202~20422] |
| 4 | 2-Br | -0.07660 | 91.8 | 179~181 | 179~182[ |
| 5 | 3-NO2 | -0.10429 | 94.7 | 201~203 | 200~203[ |
| 6 | 3-Cl | -0.07463 | 92.1 | 184~186 | 186~187[ |
| 7 | 3-Br | -0.07444 | 91.6 | 226~228 | 227~228[ |
| 8 | 3-CH3 | -0.06000 | 88.1 | 204~206 | 204[ |
| 9 | 3-CH3O | -0.05980 | 86.9 | 156~158 | 157~158[ |
| 10 | 3-OH | -0.05253 | 86.2 | 224~226 | 223~225[ |
| 11 | 4-NO2 | -0.11459 | 97.3 | 198~200 | 198~200[ |
| 12 | 4-Cl | -0.07305 | 91.7 | 199~202 | 198~201[ |
| 13 | 4-Br | -0.07019 | 90.8 | 194~197 | 195~197[ |
| 14 | 4-CH3 | -0.05901 | 86.7 | 232~234 | 233~234[ |
| 15 | 4-CH3O | -0.05166 | 85.9 | 189~191 | 188~190[ |
| 16 | 4-OH | -0.05108 | 84.5 | 184 | 182[ |
| Atom | Charge | Atom | Charge |
|---|---|---|---|
| Zn | 0.696 | N1 | -0.310 |
| S | 1.245 | N2 | -0.156 |
| O1 | -0.520 | N3 | -0.180 |
| O2 | -0.525 | N4 | -0.171 |
| O3 | -0.243 | N5 | -0.170 |
| O4 | -0.072 | N6 | -0.276 |
| Atom | Charge | Atom | Charge |
|---|---|---|---|
| Zn | 0.696 | N1 | -0.310 |
| S | 1.245 | N2 | -0.156 |
| O1 | -0.520 | N3 | -0.180 |
| O2 | -0.525 | N4 | -0.171 |
| O3 | -0.243 | N5 | -0.170 |
| O4 | -0.072 | N6 | -0.276 |
| [1] |
doi: 10.1002/slct.v2.23 |
| [2] |
|
| [3] |
doi: 10.3390/app12052710 |
| [4] |
doi: 10.1002/jhet.v55.3 |
| [5] |
doi: 10.3390/12122621 |
| [6] |
doi: 10.1016/j.cclet.2010.03.022 |
| [7] |
doi: 10.1016/j.tetlet.2012.10.029 |
| [8] |
doi: 10.1007/s11164-013-1071-x |
| [9] |
doi: 10.1016/j.tetlet.2015.06.004 |
| [10] |
doi: 10.1016/j.tetlet.2019.151587 |
| [11] |
doi: 10.33263/LIANBS |
| [12] |
doi: 10.1016/j.poly.2018.09.002 |
| [13] |
doi: 10.3390/cryst13121681 |
| [14] |
doi: 10.1016/j.jssc.2021.122610 |
| [15] |
doi: 10.1016/j.apsusc.2022.153250 |
| [16] |
doi: 10.1016/j.poly.2018.03.006 |
| [17] |
doi: 10.1016/j.ccr.2010.10.038 |
| [18] |
doi: 10.1039/c3ce41157e |
| [19] |
|
|
(朱为宏, 杨雪艳, 李晶, 有机波谱及性能分析法, 化学工业出版社, 北京 2007, p. 55.)
|
|
| [20] |
doi: 10.1002/ajoc.v1.4 |
| [21] |
|
| [22] |
doi: 10.1007/s11164-016-2634-4 |
| [23] |
|
|
(谢宗波, 张士国, 姜国芳, 梁萌, 乐长高, 有机化学, 2017, 32, 14.)
|
|
| [24] |
|
|
(段建凤, 穆小静, 周曌, 毕旌富, 肖尚友, 化学通报, 2018, 81, 1023.)
|
|
| [25] |
doi: 10.1016/j.tet.2024.133915 |
| [26] |
doi: 10.6023/cjoc201903025 |
|
(张晓鹏, 朱妍洁, 朱奕崧, 李政伟, 张贵生, 有机化学, 2019, 39, 2392.)
doi: 10.6023/cjoc201903025 |
|
| [27] |
doi: 10.1039/C8RA07256F |
| [28] |
|
| [29] |
doi: 10.1107/S0021889808042726 |
| [30] |
|
| [1] | 张元贺, 沈运杰, 谈东兴, 韩福社. Cryptoquinonemethides合成中螺五环非对映异构体环丙烷化反应活性及选择性研究[J]. 有机化学, 2026, 46(1): 96-105. |
| [2] | 李金霞, 邓远程, 李嘉瑜, 郭益豪, 王广凤, 段阿冰, 瞿双林. 钯催化硅环开环/交叉偶联反应机理研究[J]. 有机化学, 2025, 45(8): 2938-2944. |
| [3] | 张继东, 杨垚, 张杰, 厍伟. 基于聚集诱导效应(AIE)-激发态分子内质子转移(ESIPT)效应的四苯乙烯荧光探针对Zn(II)检测研究[J]. 有机化学, 2024, 44(4): 1337-1342. |
| [4] | 孙庆浩, 鲍晓光. 钼催化芳香醛脱氧偶联反应机制的理论研究[J]. 有机化学, 2024, 44(11): 3518-3525. |
| [5] | 李泽辉, 邹昊宇, 李林才, 赵怡玲, 朱红平. N,O-配体钴化合物的合成及其环氧丙烷羰化酯化的催化性能[J]. 有机化学, 2023, 43(11): 3907-3915. |
| [6] | 马丽文, 魏晓叶, 赵紫琳, 赵昂, 邓祥文, 霍丙南, 马刚, 张春芳. 端炔偶联反应中铜变价催化机制的理论研究[J]. 有机化学, 2022, 42(6): 1811-1819. |
| [7] | 丁思懿, 祖维赛, 苗宗成, 徐亮. 8-氨基喹啉骨架螯合的四配位B,B-二芳基络合物的合成与计算研究[J]. 有机化学, 2022, 42(3): 812-818. |
| [8] | 徐曼, 夏远志. 铑(III)催化N-苯氧基乙酰胺与亚甲基氧杂环丁酮氧化还原中性的碳氢活化/环化反应的机理研究[J]. 有机化学, 2021, 41(8): 3272-3278. |
| [9] | 黄利, 王毓浩, 刘吉英, 李世俊, 张文静, 蓝宇. 铜催化异氰酸酯加成反应机理研究[J]. 有机化学, 2021, 41(11): 4347-4352. |
| [10] | 祁连山, 王涛, 魏永梅, 田恒水. 共还原剂醛类对金属卟啉催化丙烯环氧化的作用研究[J]. 有机化学, 2020, 40(5): 1305-1309. |
| [11] | 朱继华, 张浩, 刘敏, 刘旌江, 廖原, 权正军, 王喜存. pH调控下基于分子内电荷转移(ICT)机理的硫化氢荧光探针[J]. 有机化学, 2020, 40(4): 1043-1049. |
| [12] | 张亦伟, 陈艺林, 方霄龙, 袁友珠, 朱红平. 草酸酯加氢制乙二醇钌金属均相催化体系的研究进展[J]. 有机化学, 2017, 37(9): 2275-2286. |
| [13] | 邹晓川, 石开云, 李俊, 王跃, 王存, 邓朝芳, 任彦荣, 谭君, 傅相锴. 不同价态Mn(II, III, V)催化的烯烃环氧化反应研究进展[J]. 有机化学, 2016, 36(8): 1765-1778. |
| [14] | 苏碧云, 郏佩瑜, 王彦昭, 李亚宁, 黄鹤, 李谦定. 膦基苯磺酸(PO)金属配合物催化乙烯与极性单体的共聚反应及其机理研究进展[J]. 有机化学, 2016, 36(10): 2344-2352. |
| [15] | 刘睿, 肖树萌, 钟向宏, 曹育才, 梁胜彪, 刘振宇, 叶晓峰, 沈安, 朱红平. 基于[PNP]配体的铬催化剂体系选择性催化乙烯齐聚的研究进展[J]. 有机化学, 2015, 35(9): 1861-1888. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||