综述与进展
冯凯a, 贺兆波*,a, 王震*,a,b
收稿日期:2025-09-28
修回日期:2025-11-28
Feng, Kaia, He, ZhaoBo*,a, Wang, Zhen*,a,b
Received:2025-09-28
Revised:2025-11-28
Contact:
*E-mail: 文章分享
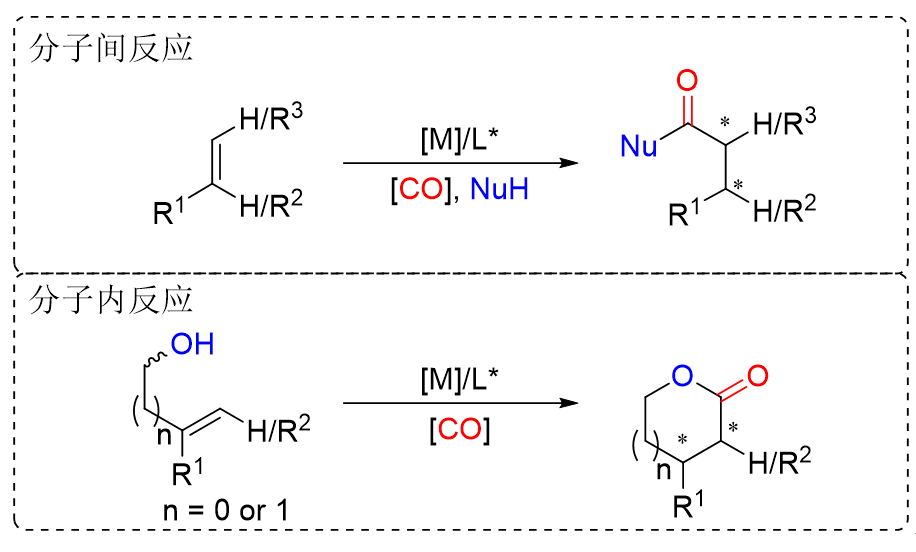
不对称氢酯基化反应因其原子经济性和步骤简洁性,已成为合成手性羧酸衍生物最具吸引力的途径之一。该反应的核心挑战在于同时实现高化学、区域与对映体选择性的协同调控,而手性配体作为催化体系的关键组分,通过精准调控过渡金属活性中心的立体电子特性及配位环境,可以直接主导反应的催化效率与立体化学调控。本综述系统梳理了数十年来钯催化烯烃的不对称氢酯基化反应研究进展,同时评述了手性配体的创新设计对选择性控制的突破性贡献,并展望了该领域面临的挑战与未来发展方向。
冯凯, 贺兆波, 王震. 钯催化烯烃的不对称氢酯基化反应研究进展[J]. 有机化学, doi: 10.6023/cjoc202509035.
Feng, Kai, He, ZhaoBo, Wang, Zhen. Recent Advances in Palladium-Catalyzed Asymmetric Hydroesterification of Alkenes[J]. Chinese Journal of Organic Chemistry, doi: 10.6023/cjoc202509035.
| [1] (a) Moen M.D.; Keam, S.J.CNS Drugs2009, 23, 1057. (b) Butler A.R.; Wu, Y.-L.Chem.Soc.Rev.1992, 21, 85. (c) Xia J.Y.; Yang D.Q.; Long, Y.H.Chin.J.Org.Chem.2011, 31, 593 (in Chinese). (夏九云, 杨定乔, 龙玉华,有机化学, 2011, 31, 593). (d) Wang J.G.; Xu C.C.; Wong Y.K.; Li Y.J.; Liao F.L.; Jiang T.L.; Tu Y.Y.Engineering2019, 5, 32. (e) Halama A.; Zapadlo, M.Org.Process Res.Dev.2019, 23, 102. (f) Ma K.Q.; Martin B.S.; Yin X.L.; Dai, M.J.Nat.Prod.Rep.2019, 36, 174. (g) Iacoviello M.; Palazzuoli A.; Gronda, E. Eur.J.Clin.Invest.2021, 51, e13624. (h) Li J.H.; Shi, Y.A.Chem.Soc.Rev.2022, 51, 6757. (i) Kuai, C -S.; Wang Y.R.; Yang T.; Wu, X -F.J.Am.Chem.Soc.2025, 147, 7950. [2] (a) Yang, H.; Tang, W.J.Chem.Rec.2020, 20, 23. (b) Mas-Roselló J.; Herraiz A.G.; Audic B.; Laverny A.; Cramer, N.Angew.Chem.Int.Ed.2021, 60, 13198. (c) Margalef J.; Biosca M.; de la Cruz Sánchez, P.; Faiges J.; Pàmies O.; Diéguez, M.Coord.Chem.Rev.2021, 446, 214120. (d) Wang, H.; Wen, J.L.; Zhang, X.M. Chem.Rev.2021, 121, 7530. [3] (a) Knowles W.S.; Sabacky, M.J.J.Chem.Soc., Chem.Commun.1968, 20, 1445. (b) Horner L.; Sieged H.; Buthe, H.Angew.Chem.Int.Ed.1968, 7, 942. (c) Ni H.Z.; Chan W.-L.; Lu, Y.X.Chem.Rev.2018, 118, 9344. (d) Xu G.Q.; Senanayake C.H.; Tang, W.J.Acc.Chem.Res.2019, 52,1101. (e) Imamoto, T. Proc.Jpn.Acad., Ser.B 2021, 97, 520. (f) Stephan M.; Jeran M.; Mohar, B. Org.Process Res.Dev.2024, 28, 11. (g) Chen W.; Cai P.; Zhou H.C.; Madrahimov, S.T.Angew.Chem.Int.Ed.2024, 63, e202315075. (h) Su, L.; Gao, S.; Liu, J.W.Nat.Commun.2024, 15, 7248. (i) Ye B.H.; Su L.; Zheng K.T.; Gao S.; Liu, J.W.Angew.Chem.Int.Ed.2025, 64, e202413949. [4] (a) Zhou, Y.R.; Gong, Y.F.Eur.J.Org.Chem.2011, 6092. (b) Shen X.Z.; Chen X.; Chen J.P.; Sun Y.F.; Cheng Z.Y.; Lu Z.Nat.Commun.2020, 11, 783. (c) Liu,W.; Jiang Q.W.; Yang, X.Y.Angew.Chem.Int.Ed.2020, 59, 23598. (d) Wu, X Q.; Turlik A.T.; Luan B.X.; He F.; Qu J.P.; Houk K.N.; Chen.Y.F.Angew.Chem.Int.Ed.2022, 61, e202207536. (e) Wu, X Q.; Qu J.P.; Chen.Y.F.J.Am.Chem.Soc.2020, 142, 15654. (f) Wu, X Q.; Li H.Y.; He F.; Qu J.P.; Chen.Y.F.Chin.J.Chem.2023, 41, 1673. (g) Jin L.; Li Y.; Mao Y.H.; He X.-B.; Lu Z.; Zhang Q.; Shi B.-F.Nat.Commun.2024, 15, 4908. (h) Cui B.; Zheng Y.T.; Sun H.; Shang H.J.; Du M.; Shang Y.X.; Yavuz, C.T.Nat.Commun.2024, 15, 6647. [5] (a) Hiroi K.; Sone, T.Curr.Org.Synth.2008, 5, 305. (b) Liu Z.Q.; Feng X.Q.; Du, H.F.Org.Lett.2012, 14, 3154. (c) Trost B.M.; Rao, M.Angew.Chem.Int.Ed.2015, 54, 5026. (d) Otocka S.; Kwiatkowska M.; Madalinska L.; Kiełbasiński P.Chem.Rev.2017, 117,4147. (e) Li X.; Zheng S.-C.; Zhao, X.-M.Eur.J.Org.Chem.2025, 28, e202401172. [6] (a) Su B.; Zhou T.-G.; Xu P.-L.; Shi Z.-J.; Hartwig, J.F.Angew.Chem.Int.Ed.2017, 56,7205. (b) Babu K .N.; Kinthada L.K.; Das P.P.; Bisai A.Chem.Commun.2018, 54, 7963. (c) Zhang S.T.; Wu S.J.; Wang Q.; Xu S.J.; Han Y.; Yan C.-G.; Zhang J.L.; Wang, L.Angew.Chem.Int.Ed.2023, 62, e202300309. (d) Tu Y.S.; Xu B.; Wang Q.; Dong H.L.; Zhang Z.-M.; Zhang, J.L.J.Am.Chem.Soc.2023, 145, 4378. (e) Liao G.; Shi, B,-F. Acc.Chem.Res.2025, 58, 1562. (f) Yan Xu.; Feng W.; Ng J.X.; Li J.X.; Liu Y.; Zhang B.; Shao P.-L.; Zhao, Y.Chem.Soc.Rev.2025, Advance Article. [7] (a) Lin, G.-Q.; Li, Y.-M.; Chan, A.S.C.Wiley, New York, 2001. (b) Xie J.-H.; Zhou, Q.-L.Acta Chim.Sinica2012, 70, 1427. (c) Zhao, W.X.; Yang, D.Y.; Zhang, Y.H.Chin.J.Org.Chem.2016, 36, 2301. [8] (a) Yu X.H.; Wang, W.Chem.Asian J.2008, 3, 516. (b) Lyubimov S.E.; Kalinin V.N.; Tyutyunov A.A.; Olshevskaya V.A.; Dutikova Y.V.; Cheong C.S.; Petrovskii P.V.; Safronov A.S.; Davankov V.A.Chirality2009, 21, 2. (c) Luo J.; Zhang T.; Wang L.; Liao G.; Yao Q.J.; Wu Y.J.; Zhan B.B.; Lan Y.; Lin X.F.; Shi, B.F.Angew.Chem.Int.Ed.2019, 58, 6708. (d) Liu X.-R.; Cui, P -F.; Guo S.-T.; Lin Y.-J.; Jin, G.-X.J.Am.Chem.Soc.2021, 143, 5099. (e) Li P.P.; Zheng E.; Li G.L.; Luo Y.C.; Huo X.H.; Ma S.M.; Zhang W.B.Science2024, 385, 972. [9] (a) Ren X.Y.; Wang Z.; Shen C.R.; Tian X.X.; Tang L.; Ji X.L.; Dong, K.W.Angew.Chem.Int.Ed.2021, 60,17693. (b) Wang, Z.; Shen, C.R.; Dong, K.W.Angew.Chem.Int.Ed.2024, e202410967. (c) Wang Z.; Dong, K.W.Chin.J.Org.Chem.2024, 44, 3249 (in Chinese). (王震, 董开武, 有机化学, 2024, 44, 3249). [10] (a) Clegg, W.; Elsegood, M.R.J.; Eastham, G.R.; Tooze, R.P.; Wang, X.L.; Whiston, K.Chem.Commun.1999, 1877. (b) Kiss, G.Chem.Rev. 2001, 101, 3435. (c) del Rıo I.; Ruiz N.; Claver, C.Inorg. Chem.Commun.2000, 3, 166. (d) Godard, C.; Muñoz, B.K.; Ruiz.A.; Claver, C.Dalton Trans.2008, 853. [11] (a) Xu Z.S.; Shen C.R.; Zhang H.R.; Wang P.; Dong, K.W.Org.Chem.Front.2021, 8, 1163. (b) Peng J.-B.; Liu X.-L.; Li L.; Wu, X.-F.Sci.China: Chem.2022, 65, 441. (c) Cheng S.D.; Luo Y.; Yu T.; Li J.; Gan C.F.; Luo S.; Zhu Q.ACS Catal.2022, 12, 837. (d) Shen C.R.; Dong K.W.Synlett2022, 33, 815. [12] (a) Botteghi C.; Consiglio G.; Pino P.Chimia1973, 27, 477. (b) Consiglio G.; Pino P.Chimia1976, 30, 193. (c) Consiglio, G.Helv.Chim.Acta. 1976, 59, 124. (d) Consiglio, G.J.Organomet.Chem. 1977, 132, C26. [13] Hayashi T.; Tanaka M.; Ogata I.Tetrahedron Lett.1978, 41, 3925. [14] Cometti G.; Chiusoli, G.P.J.Organomet.Chem.1982, 236, C31. [15] Zhou H.Y.; Hou J.G.; Cheng J.; Lu S.J.; Fu.H.X.; Wang, H.Q.J.Organomet.Chem.1997, 543, 227. [16] Oi S.; Nomura M.; Aiko T.; Inoue, Y.J.Mol.Catal.A: Chem.1997, 115, 289. [17] Beller M.; Comils B.; Frohning C.D.; Kohlpaintner, C.W.J.Mol.Catal.A: Chem.1995, 104, 17. [18] Nozaki K.; Kantam M.L.; Horiuchi T.; Takaya, H.J.Mol.Catal.A: Chem.1997, 118, 247. [19] Wang L.L.; Kwok W.H.; Chan A.S.; Tu T.; Hou, X.L; Dai, L.X. Tetrahedron: Asymmetry 2003, 14, 2291. [20] Kawashima Y.; Okano K.; Nozaki K.; Hiyama, T.Bull.Chem.Soc.Jpn.2004, 77, 347. [21] Muñoz.B.K.; Marinetti A.; Ruiz A.; Castillon S.; Claver, C.Inorg.Chem.Commun.2005, 8, 1113. [22] Marinetti A.; Kruger V.; Buzin, F.-X.Coord.Chem.Rev.1998, 178-180, 755. [23] Muñoz B.K.; Godard C.; Marinetti A.; Ruiz A.; Benet-Buchholz J.; Claver C.Dalton Trans.2007, 47, 5524. [24] Godard C.; Ruiz A.; Claver.C.Helv.Chimica Acta 2006, 89, 1610. [25] Guiu E.; Caporali M.; Munoz B.; Müller C.; Lutz M.; Spek A.L.; van Leeuwen, P.W.Organometallics 2006, 25, 3102. [26] Yao Y.-H.; Zou X.-J.; Wang Y.; Yang H.-Y.; Ren, Z.-H; Guan Z.-H.Angew.Chem.Int.Ed.2021, 60, 23117. [27] Chelucci G.; Cabras M.A.; Botteghi C.; Chelucci M.Tetrahedron: Asymmetry1994, 5, 299. [28] Konrad T.M.; Fuentes J.A.; Slawin A.M.Z.; Clarke, M.L.Angew.Chem.Int.Ed.2010, 49, 9197. [29] Konrad T.M.; Durrani J.T.; Cobley C.J.; Clarke, M.L.Chem.Commun.2013, 49, 3306. [30] Gallarati S.; Dingwall P.; Fuentes J.A.; Bühl M.; Clarke M.L.Organometallics2020, 39, 4544. [31] Harkness G.J.; Clarke M.L.Eur.J.Org.Chem.2017, 4859. [32] Li J.F.; Chang W.J.; Ren W.L.; Dai J.; Shi Y.A.Org.Lett.2016, 18, 5456. [33] Li J.F.; Ren W.L.; Dai J.; Shi, Y.A.Org.Chem.Front.2018, 5, 75. [34] Pongrácz P.; Seni A.A.; L.T.; Kollár L.Mol.Catal.2017, 438, 15. [35] Ji X.L.; Shen C.R.; Tian X.X.; Dong, K.W.Org.Lett.2021, 23, 8645. [36] Zhou H.Y.; Lu S.J.; Hou J.G.; Chen J.; Fu H.X.; Wang H,Q.Chem.Lett.1996, 339. [37] Blanco C.; Ruiz A.; Godard C.; Fleury-Bregeot N.; Marinetti A.; Claver, C.Adv.Synth.Catal.,2009, 351,1813. [38] Andrieu J.; Braunstein P.; Naud F.; Adams, R.D.J.Organomet.Chem.2000, 601, 43. [39] Fuentes J A.; Durrani J T.; Leckie S M.; Crawford L.; Bühl M.; Clarke M.L.Catal.Sci.Technol.2016, 6, 7477. [40] (a) Castarlenas R.;Di Giuseppe A.; Pérez-Torrente J.J.; Oro, L.A.Angew.Chem.Int.Ed.2013, 52, 211. (b) Ai, H J.; Lu W.Y.; Wu, X.-F.Angew.Chem.Int.Ed.2021, 60, 17178. (c) Wang L.-C.; Wu X.-F.Nat.Commun.2025, 16, 4663. [41] Xiao W.-J.; Alper, H.J.Org.Chem.2001, 66, 6229. [42] Wang X.H.; Wang B.; Yin X.M.; Yu W.A.; Liao Y.; Ye J.L.; Wang M.; Hu L.R.; Liao, J.Angew.Chem.Int.Ed.2019, 58, 12264. [43] Alper H.; Hamel N.J.Chem.Soc., Chem.Commun.1990, 135. [44] Yu W.-Y.; Bensimon C.; Alper, H.Chem.Eur.J.1997, 3, 417. [45] Cao P.; Zhang, X.M.J.Am.Chem.Soc.1999, 121, 7708. [46] Shi Z.L.; Shen C.R.; Dong, K.W.Chem.Eur.J.2021, 27, 18039. [47] Dong C.; Alper, H.J.Org.Chem.2004, 69, 5011. [48] Wang, H.N; Dong, B.; Wang Y.; Li J.F.; Shi, Y.A.Org.Lett.2014, 16, 186. [49] Li J.F.; Chang W.J.; Ren W.L.; Liu W.; Wang H.N.; Shi, Y.A.Org.Biomol.Chem.2015, 13, 10341. [50] Tian D.S.; Xu R.H.; Zhu J.B.; Huang J.X.; Dong W.; Claverie J.; Tang, W.J.Angew.Chem.Int.Ed.2021, 60, 6305. [51] Alper H.; Hamel, N.J.Am.Chem.Soc.1990, 112, 2803. [52] Jiang B.; Huang Z.-G.;Cheng K.-J.Heterocycles2004, 63, 2797. [53] Miquel-Serrano M.D.; Aghmiz A.; Die´guez M.; Masdeu-Bulto´ A.M.; Claver C.; Sinou D.Tetrahedron: Asymmetry1999, 10, 4463. [54] Huang Z.J.; Cheng Y.Z.; Chen X.P.; Wang H.-F.; Du C.-X.; Li, Y.H.Chem.Commun.2018, 54, 3967. [55] Ji X.L.; Shen, C.R., Tian, X.X.; Zhang H.R.; Ren X.Y.; Dong, K.W.Angew.Chem.Int.Ed.2022, 61, e202204156. [56] Marcos-Ayuso G.; Quesada D.; Cobos-Abad M.Y.; Lendínez C.; Fernández-Moyano S.; Mauleón P.; Arrayás, R.G.ACS Catal.2025, 15, 20230. |
| [1] | 赵倩倩, 魏培垚, 刘孙典, 张博鑫, 梁承远. 钯催化串联反应在含季碳中心的复杂天然产物全合成中的应用[J]. 有机化学, 2025, 45(9): 3289-3300. |
| [2] | 夏颖, 朱辰龙, 孙炳峰. 抗乙肝病毒药物恩替卡韦的全合成研究进展★[J]. 有机化学, 2025, 45(9): 3186-3202. |
| [3] | 陈奇姝, 杨泊, 稂琪伟, 丁小兵, 李秀秀, 张绪穆. 铱/f-Amphox催化吡啶酮的不对称氢化反应制备手性吡啶醇★[J]. 有机化学, 2025, 45(9): 3326-3334. |
| [4] | 吕亚, 何贵含, 刘剑剑, 陈永正. 细微差别取代化合物的手性识别及不对称催化反应研究进展★[J]. 有机化学, 2025, 45(9): 3163-3174. |
| [5] | 高峰, 张万斌. 贝达喹啉类抗结核药物的合成进展★[J]. 有机化学, 2025, 45(9): 3113-3127. |
| [6] | 武烨, 陈春霞, 彭进松. C—H键不对称羟基化反应的研究进展[J]. 有机化学, 2025, 45(8): 2726-2745. |
| [7] | 任揽星, 徐阿娜, 肖锡林, 游恒志, 宋利娟. 溶剂效应对Ir催化的不对称氢化反应对映选择性影响的理论研究[J]. 有机化学, 2025, 45(8): 2904-2912. |
| [8] | 沈健, 王彦博, 吴正兴, 徐德锋, 张万斌. α,β-不饱和γ-内酯(酰胺)的不对称催化合成[J]. 有机化学, 2025, 45(8): 2637-2659. |
| [9] | 李旻昊, 王泽溟, 黄庆, 左伟伟. 钴(II)催化的酮不对称转移氢化反应[J]. 有机化学, 2025, 45(7): 2451-2460. |
| [10] | 任天磊, 汪鑫, 丛欢. 蒽光二聚体衍生的手性单膦配体: 借助化学拆分的制备路线和不对称烯丙基胺化的合成应用[J]. 有机化学, 2025, 45(6): 2208-2221. |
| [11] | 杜一鸣, 贾均松, 李玉龙, 舒伟. 手性α-芳基酮的催化合成研究进展[J]. 有机化学, 2025, 45(6): 1838-1870. |
| [12] | 苏雷, 杨熙, 闫捷, 蒋元力, 陈丽娟, 郑庆舒, 刘家旺. 不对称羰基化偶联反应研究进展[J]. 有机化学, 2025, 45(6): 2007-2047. |
| [13] | 王霜, 毛羊杰, 娄绍杰, 许丹倩. 基于氧化型导向基团的不对称C—H键官能团化反应研究进展[J]. 有机化学, 2025, 45(6): 1961-1994. |
| [14] | 周强, 杨宝臻, 郝贵林, 罗木鹏, 曹石, 赵蓓, 袁华, 王守国. 铑(III)催化的非活化烯烃与α-重氮羰基化合物的对映选择性烯丙位C—H键烷基化反应[J]. 有机化学, 2025, 45(6): 2109-2120. |
| [15] | 唐梦瑶, 杨晓瑜. 手性磷酸催化不对称亲电胺化反应研究进展[J]. 有机化学, 2025, 45(6): 1785-1818. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||