有机化学 ›› 2011, Vol. 31 ›› Issue (12): 2061-2066. 上一篇 下一篇
研究论文
孙亮1,凌云1,王灿1,孙玉凤1,芮昌辉2,杨新玲*,1
Sun Liang1 Ling Yun1 Wang Can1 Sun Yufeng1 Rui Changhui2 Yang Xinling*,1
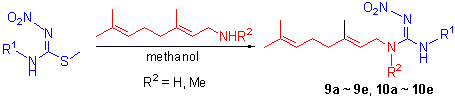
以蚜虫报警信息素的主要成分E-β-法尼烯(E-β-farnesene, 简称EBF)为先导, 分别用不同取代硝基胍替代EBF中的共轭双键, 设计合成了10个未见文献报道的EBF类似物, 其结构均经1H NMR, IR和HRMS分析确证, 并对化合物的生物活性进行了测试, 分析比较了不同取代硝基胍的生物活性差异. 生物活性试验结果表明, 目标化合物对桃蚜(Myzus persicae)均具有一定的生物活性, 其中化合物9a~9e的活性优于E-β-法尼烯.