有机化学 ›› 2021, Vol. 41 ›› Issue (3): 1153-1160.DOI: 10.6023/cjoc202009056 上一篇 下一篇
研究论文
兰新婵1, 王丽丽1,*( ), 段征1,*(
), 段征1,*( ), François Mathey1
), François Mathey1
收稿日期:2020-09-28
修回日期:2020-11-03
发布日期:2020-11-12
通讯作者:
王丽丽, 段征
基金资助:
Xinchan Lan1, Lili Wang1,*( ), Zheng Duan1,*(
), Zheng Duan1,*( ), François Mathey1
), François Mathey1
Received:2020-09-28
Revised:2020-11-03
Published:2020-11-12
Contact:
Lili Wang, Zheng Duan
About author:Supported by:文章分享

芘具有良好的刚性平面和高的荧光量子产率, 是有机光电材料研究中重要的结构单元, 由于其电子结构特点, 芘的2位官能团化非常困难. 利用易得的1-芘醇和H-亚磷酸酯或有机磷氯化合物合成了一系列1-芘基磷酸酯化合物, 经磷杂Fries重排反应在温和条件下实现了芘2位的磷酰化, 并得到相应的2-磷酰基芘衍生物. 该类化合物还为新型含磷共轭化合物芘并1,3-氧杂磷杂环戊二烯的研究奠定了基础. 本研究不但为芘类化合物的官能团化提供了一个新方法, 而且为新型含磷共轭化合物的设计与合成开辟了新路线, 并对合成的新型芘类化合物的紫外吸收和荧光发射等性质进行了初步测定.
兰新婵, 王丽丽, 段征, François Mathey. 磷杂Fries重排反应用于合成2-芘基膦化合物[J]. 有机化学, 2021, 41(3): 1153-1160.
Xinchan Lan, Lili Wang, Zheng Duan, François Mathey. Synthesis of 2-Pyrenylphosphines via Phospho-Fries Rearrangement[J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 1153-1160.
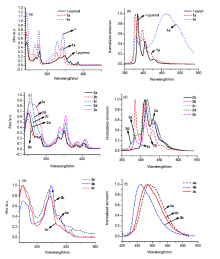

| Compound | λabsa/nm | λex/nm | λema/nm | τb/ns | Φfc | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1-Pyrenol | 335, 347, 364, 384 | 280 | 385, 405, 428 | 14.0 | 0.38 | ||||
| 1a | 326, 342 | 280 | 378, 398, 418 | 25.1 | 0.23 | ||||
| 1b | 326, 342 | 280 | 379, 398, 418 | 23.8 | 0.19 | ||||
| 1c | 326, 342 | 280 | 378, 397, 417 | 27.0 | 0.23 | ||||
| 1d | 329, 345 | 280 | 380, 399, 419 | 16.9 | 0.23 | ||||
| Compound | λabsa/nm | λex/nm | λema/nm | τb/ns | Φfc | ||||
| 1e | 328, 344 | 280 | 379, 398, 462 | 24.8 | 0.16 | ||||
| 2a | 335, 351, 383, 405 | 280 | 411, 433, 456 | 9.1 | 0.39 | ||||
| 2b | 335, 350, 384, 405 | 280 | 385, 408, 431, 457 | 10.6 | 0.39 | ||||
| 2c | 335, 351, 387, 408 | 280 | 415,438, 463 | 9.1 | 0.40 | ||||
| 2d | 338, 354, 385, 407 | 280 | 397, 418, 439 | 7.8 | 0.004 | ||||
| 2e | 338, 355, 386, 409 | 280 | 386, 418, 439 | 2.0 | 0.10 | ||||
| 4a | 254, 342 | 310 | 471 | 7.7 | 0.04 | ||||
| 4b | 252, 348 | 330 | 461 | 2.7 | 0.11 | ||||
| 4c | 249, 345 | 310 | 463 | 3.4 | 0.13 | ||||
| Compound | λabsa/nm | λex/nm | λema/nm | τb/ns | Φfc | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1-Pyrenol | 335, 347, 364, 384 | 280 | 385, 405, 428 | 14.0 | 0.38 | ||||
| 1a | 326, 342 | 280 | 378, 398, 418 | 25.1 | 0.23 | ||||
| 1b | 326, 342 | 280 | 379, 398, 418 | 23.8 | 0.19 | ||||
| 1c | 326, 342 | 280 | 378, 397, 417 | 27.0 | 0.23 | ||||
| 1d | 329, 345 | 280 | 380, 399, 419 | 16.9 | 0.23 | ||||
| Compound | λabsa/nm | λex/nm | λema/nm | τb/ns | Φfc | ||||
| 1e | 328, 344 | 280 | 379, 398, 462 | 24.8 | 0.16 | ||||
| 2a | 335, 351, 383, 405 | 280 | 411, 433, 456 | 9.1 | 0.39 | ||||
| 2b | 335, 350, 384, 405 | 280 | 385, 408, 431, 457 | 10.6 | 0.39 | ||||
| 2c | 335, 351, 387, 408 | 280 | 415,438, 463 | 9.1 | 0.40 | ||||
| 2d | 338, 354, 385, 407 | 280 | 397, 418, 439 | 7.8 | 0.004 | ||||
| 2e | 338, 355, 386, 409 | 280 | 386, 418, 439 | 2.0 | 0.10 | ||||
| 4a | 254, 342 | 310 | 471 | 7.7 | 0.04 | ||||
| 4b | 252, 348 | 330 | 461 | 2.7 | 0.11 | ||||
| 4c | 249, 345 | 310 | 463 | 3.4 | 0.13 | ||||
| [1] |
(a) Figueira-Duarte, T. M.; Müllen, K. Chem. Rev. 2011, 111, 7260.
pmid: 31909994 |
|
(b) Lorbach, D.; Wagner, M.; Baumgarten, M.; Müllen, K. Chem. Commun. 2013, 49, 10578.
pmid: 31909994 |
|
|
(c) Zhang, S. Q.; Qiao, X. L.; Chen, Y.; Wang, Y. Y.; Edkins, R. M.; Liu, Z. Q.; Li, H. X.; Fang, Q. Org. Lett. 2014, 16, 342.
pmid: 31909994 |
|
|
(d) Mateo-Alonso, A. Chem. Soc. Rev. 2014, 43, 6311.
pmid: 31909994 |
|
|
(e) Cariati, E.; Botta, C.; Danelli, S. G.; Forni, A.; Giaretta, A.; Giovanella, U.; Lucenti, E.; Marinotto, D.; Righetto, S. Ugo, R. Chem. Commun. 2014, 50, 14225.
pmid: 31909994 |
|
|
(f) Liu, J. Z.; Narita, A.; Osella, S.; Zhang, W.; Schollmeyer, D.; Beljonne, D.; Feng, X. L.; Müllen, K. J. Am. Chem. Soc. 2016, 138, 2602.
doi: 10.1021/jacs.5b10399 pmid: 31909994 |
|
|
(g) Krzeszewski, M.; Sahara, K.; Poronik, Y. M.; Kubo, T.; Gryko, D. T. Org. Lett. 2018, 20, 1517.
pmid: 31909994 |
|
|
(h) Fu, C. X.; Luo, S.; Li, Z. Z..; Ai, X.; Pang, Z. G.; Li, C.; Chen, K.; Zhou, L.; Li, F.; Huang, Y.; Lu, Z. Y. Chem. Commun. 2019, 55, 6317.
pmid: 31909994 |
|
|
(i) Ascherl, L.; Evans, E. W.; Gorman, J.; Orsborne, S.; Bessinger, D.; Bein, T.; Friend, R. H.; Auras, F. J. Am. Chem. Soc. 2019, 141, 15693.
pmid: 31909994 |
|
|
(j) Shao, J.-Y.; Yang, N.; Guo, W.; Cui, B.-B.; Chen, Q.; Zhong, Y.-W. Chem. Commun. 2019, 55, 13406.
pmid: 31909994 |
|
|
(k) Takaishi, K.; Iwachidoo, K.; Ema, T. J. Am. Chem. Soc. 2020, 142, 1774.
pmid: 31909994 |
|
|
(l) Wang, B. B.; Zhang, F.; Wang, S. K.; Yang, R. J.; Chen, C. C.; Zhao, W. Chem. Commun. 2020, 56, 2598.
pmid: 31909994 |
|
|
(m) Chen, Y.; Wang, Y. Y.; Yu, P.; Liu, Z. Q.; Fang, Q. Chin. J. Org. Chem. 2012, 32, 589. (in Chinese).
pmid: 31909994 |
|
|
(陈莹, 王园园, 于萍, 刘志强, 方奇, 有机化学, 2012, 32, 589.)
pmid: 31909994 |
|
|
(n) Zhong, K. L.; Guo, B. F.; Zhou, X.; Cai, K. D.; Tang, L. J.; Jin, L. Y. Prog. Chem. 2015, 27, 1230. (in Chinese).
pmid: 31909994 |
|
|
(钟克利, 郭宝峰, 周雪, 蔡克迪, 汤立军, 金龙一, 化学进展, 2015, 27, 1230.)
pmid: 31909994 |
|
|
(o) Gong, Y. B.; Zhan, X. J.; Li, Q. Q.; Li, Z. Sci. China: Chem. 2016, 59, 1623.
pmid: 31909994 |
|
| [2] |
(a) Hu, J.; Yip, J. H. K.; Ma, D.-L.; Wong, K.-Y.; Chung, W.-H. Organometallics 2009, 28, 51.
pmid: 30475600 |
|
(b) Hu, J. A.; Nguyen, M.-H.; Yip, J. H. K. Inorg. Chem. 2011, 50, 7429.
pmid: 30475600 |
|
|
(c) Deng, M.; Schley, N. D.; Ung, G. Inorg. Chem. 2018, 57, 15399.
pmid: 30475600 |
|
| [3] |
(a) Chua, C. J.; Ren, Y.; Baumgartner, T. Organometallics 2012, 31, 2425.
pmid: 28762408 |
|
(b) Behm, K.; Essner, J. B.; Barnes, C. L.; Baker, G. A.; Walensky, J. R. Dalton Trans. 2017, 46, 10867.
pmid: 28762408 |
|
| [4] |
(a) Akasaka, K.; Suzuki, T.; Ohrui, H.; Meguro, H. Anal. Lett. 1987, 20, 731.
pmid: 8898309 |
|
(b) Akasaka, K.; Ohata, A.; Ohrui, H.; Meguro, H. J. Chromatogr. B 1995, 665, 37.
pmid: 8898309 |
|
|
(c) Ohshima, T.; Hopia, A.; German, J. B.; Frankel, E. N. Lipids 1996, 31, 1091.
pmid: 8898309 |
|
|
(d) Shioji, K.; Oyama, Y.; Okuma, K.; Nakagawa, H. Bioorg. Med. Chem. Lett. 2010, 20, 3911.
pmid: 8898309 |
|
|
(e) Fang, Q. Y.; Li, J. W.; Li, S. Y.; Duan, R. H.; Wang, S. Q.; Yi, Y. P.; Guo, X. D.; Qian, Y.; Huang, W.; Yang, G. Q. Chem. Commun. 2017, 53, 5702.
pmid: 8898309 |
|
|
(f) Xing, C.; Liu, J. X.; Chen, F.; Li, Y. Y.; Lv, C. J.; Peng, Q. C.; Hou, H. W.; Li, K. Chem. Commun. 2020, 56, 4220.
doi: 10.1039/D0CC01031F pmid: 8898309 |
|
| [5] |
(a) Oyamada, T.; Sasabe, H.; Adachi, C.; Murase, S.; Tominaga, T.; Maeda, C. Appl. Phys. Lett. 2005, 86, 033503.
pmid: 29170548 |
|
(b) Matsushima, T.; Adachi, C. Thin Solid Films 2008, 516, 4288.
pmid: 29170548 |
|
|
(c) Matsushima, T.; Adachi, C. J. Appl. Phys. 2008, 103, 034501.
doi: 10.1063/1.2836972 pmid: 29170548 |
|
|
(d) Mallesham, G.; Swetha, C.; Niveditha, S.; Mohanty, M. E.; Babu, N. J.; Kumar, A.; Bhanuprakash, K.; Rao, V. J. J. Mater. Chem. C 2015, 3, 1208.
pmid: 29170548 |
|
|
(e) Lin, X.; Wegner, B.; Lee, K. M.; Fusella, M. A.; Zhang, F. Y.; Moudgil, K.; Rand, B. P.; Barlow, S.; Marder, S. R.; Koch, N.; Kahn, A. Nat. Mater. 2017, 16, 1209.
pmid: 29170548 |
|
| [6] |
Qiu, L. Q.; Hu, W.; Wu, D.; Duan, Z.; Mathey, F. Org. Lett. 2018, 20, 7821.
pmid: 30520641 |
| [7] |
(a) Melvin, L. S. Tetrahedron Lett. 1981, 22, 3375.
pmid: 32319779 |
|
(b) Heinicke, J.; Böhle, I.; Tzschach, A. J. Organomet. Chem. 1986, 317, 11.
pmid: 32319779 |
|
|
(c) Dhawan, B.; Redmore, D. J. Org. Chem. 1991, 56, 833.
pmid: 32319779 |
|
|
(d) Li, S. S.; Wang, G. Q. Phosphorus Sulfur Relat. Elem. 1991, 61, 119.
pmid: 32319779 |
|
|
(e) Paladino, J.; Guyard, C.; Thurieau, C.; Fauchère, J.-L. Helv. Chim. Acta 1993, 76, 2465.
pmid: 32319779 |
|
|
(f) Whisler, M. C.; MacNeil, S.; Snieckus, V.; Beak, P. Angew. Chem.. Int. Ed. 2004, 43, 2206.
pmid: 32319779 |
|
|
(g) Jayasundera, K. P.; Watson, A. J.; Taylor, C. M. Tetrahedron Lett. 2005, 46, 4311.
pmid: 32319779 |
|
|
(h) Yilmaz, E.; Bier, D.; Guillory, X.; Briels, J.; Ruiz-Blanco, Y. B.; Sanchez-Garcia, E.; Ottmann, C.; Kaiser, M. Chem.-Eur. J. 2018, 24, 13807.
pmid: 32319779 |
|
|
(i) Korb, M.; Lang, H. Chem. Soc. Rev. 2019, 48, 2829.
pmid: 32319779 |
|
|
(j) Patel, J. J.; Blackburn, T.; Alessi, M.; Sawinski, H.; Snieckus, V. Org. Lett. 2020, 22, 3860.
pmid: 32319779 |
|
|
(k) Taylor, C. M.; Watson, A. J. Curr. Org. Chem. 2004, 8, 623.
doi: 10.2174/1385272043370717 pmid: 32319779 |
|
| [8] |
Kenner, G. W.; Williams, N. R. J. Chem. Soc. 1955,522.
pmid: 18127479 |
| [9] |
(a) Heinicke, J.; Tzschach, A. Z. Chem. 1980, 20, 342.
|
|
(b) Heinicke, J.; Tzschach, A. Phosphorus Sulfur Relat. Elem. 1985, 25, 345.
|
|
| [10] |
(a) Washington, M. P.; Gudimetla, V. B.; Laughlin, F. L.; Deligonul, N.; He, S.; Payton, J. L.; Simpson, M. C.; Protasiewicz, J. D. J. Am. Chem. Soc. 2010, 132, 4566.
doi: 10.1021/ja1009426 pmid: 23989307 |
|
(b) Washington, M. P.; Payton, J. L.; Simpson, M. C.; Protasiewicz, J. D. Organometallics 2011, 30, 1975.
pmid: 23989307 |
|
|
(c) Laughlin, F.Li; Deligonul, N.; Rheingold, A. L.; Golen, J. A.; Laughlin, B. J.; Smith, R. C.; Protasiewicz, J. D. Organometallics 2013, 32, 7116. 888b2ca0-aaca-41d3-9521-80be05c0be74
doi: 10.1021/om400838g pmid: 23989307 |
|
|
(d) Wu, S. S.; Rheingold, A. L.; Protasiewicz, J. D. Chem. Commun. 2014, 50, 11036.
pmid: 23989307 |
|
|
(e) Laughlin, F. L.; Rheingold, A. L.; Deligonul, N.; Laughlin, B. J.; Smith, R. C.; Higham, L. J.; Protasiewicz, J. D. Dalton Trans. 2012, 41, 12016.
pmid: 23989307 |
|
|
(f) Wu, S. S.; Deligonal, N.; Protasiewicz, J. D. Dalton Trans. 2013, 42, 14866.
pmid: 23989307 |
|
| [11] |
Yesilot, S.; Cosut, B.; Alidagi, H. A.; Hacivelioglu, F.; Oezpinar, G. A.; Kilic, A. Dalton Trans. 2014, 43, 3428.
pmid: 24452362 |
| [1] | 张剑, 梁万洁, 杨艺, 闫法超, 刘会. 联烯胺化合物的区域选择性双官能团化[J]. 有机化学, 2024, 44(2): 335-348. |
| [2] | 朱彦硕, 王红言, 舒朋华, 张克娜, 王琪琳. 烷氧自由基引发1,5-氢原子转移实现C(sp3)—H键官能团化的研究进展[J]. 有机化学, 2024, 44(1): 1-17. |
| [3] | 董江湖, 宣良明, 王池, 赵晨熙, 王海峰, 严琼姣, 汪伟, 陈芬儿. 无过渡金属或无光催化剂条件下可见光促进喹喔啉酮C(3)—H官能团化研究进展[J]. 有机化学, 2024, 44(1): 111-136. |
| [4] | 刘颖杰, 石岗庆, 仇格, 张鑫, 宋冬雪, 陈宁, 于淼, 许颖. 光/电催化醚α-位官能团化研究进展[J]. 有机化学, 2023, 43(8): 2664-2681. |
| [5] | 王玉超, 刘晋彪, 何智涛. 钯催化共轭二烯的不对称氢官能团化[J]. 有机化学, 2023, 43(8): 2614-2627. |
| [6] | 许晓萍, 张翼飞, 莫小渝, 江俊. 铑催化3-重氮吲哚-2-亚胺与吡唑啉酮的C—H官能团化反应制备3-吡唑基吲哚[J]. 有机化学, 2023, 43(7): 2519-2527. |
| [7] | 潘彦年, 秦萧, 袁成凯, 鲁艺. 配体在Cp*Rh(III)催化C—H键官能团化反应中的应用[J]. 有机化学, 2023, 43(3): 924-948. |
| [8] | 侯虹宇, 程元元, 陈彬, 佟振合, 吴骊珠. 光催化烯烃α-酰化反应[J]. 有机化学, 2023, 43(3): 1012-1022. |
| [9] | 李落墨, 杨小会. 离子转移反应的研究进展[J]. 有机化学, 2023, 43(3): 1036-1044. |
| [10] | 刘宁, 爨晓丹, 李慧, 段希焱. 烯胺酮α-官能团化反应的研究进展[J]. 有机化学, 2023, 43(2): 602-621. |
| [11] | 南宁, 吴双, 秦景灏, 李金恒. 基于硅烷化启动的环化反应研究进展[J]. 有机化学, 2023, 43(10): 3414-3453. |
| [12] | 宇世伟, 陈兆华, 陈淇, 林舒婷, 何金萍, 陶冠燊, 汪朝阳. 硫代磺酸酯的合成与应用研究进展[J]. 有机化学, 2022, 42(8): 2322-2330. |
| [13] | 闫法超, 李洋, 李玉东, Mohamed Makha, 李跃辉. 氰甲基导向的吲哚选择性C—H烯基化[J]. 有机化学, 2022, 42(7): 2192-2200. |
| [14] | 王亚洲, 祝宇航, 徐丽霞, 贺瑞, 张坚. 配位导向下烯基同碳位C—H官能团化反应进展[J]. 有机化学, 2022, 42(7): 2000-2014. |
| [15] | 石宇冰, 白文己, 母伟花, 李江平, 于嘉玮, 连冰. 钯催化C—H键官能团化形成C—X (X=O, N, F, I, ……)键的密度泛函理论研究进展[J]. 有机化学, 2022, 42(5): 1346-1374. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||