有机化学 ›› 2021, Vol. 41 ›› Issue (4): 1607-1613.DOI: 10.6023/cjoc202008035 上一篇 下一篇
研究论文
成立1, 王文蓉1, 孙玉倩1, 李团结1,* ltj2008@jsnu.edu.cn., 于晨侠1, 姚昌盛1,*( )
)
收稿日期:2020-08-20
修回日期:2020-10-13
发布日期:2020-12-24
通讯作者:
李团结, 姚昌盛
基金资助:
Li Cheng1, Wenrong Wang1, Yuqian Sun1, Tuanjie Li1,* ltj2008@jsnu.edu.cn., Chenxia Yu1, Changsheng Yao1,*( )
)
Received:2020-08-20
Revised:2020-10-13
Published:2020-12-24
Contact:
Tuanjie Li, Changsheng Yao
About author:Supported by:文章分享
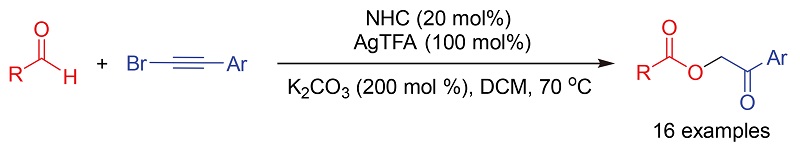
在氮杂环卡宾(NHC)/银(I)的共催化下, 以芳醛和(溴乙炔)苯及其衍生物为原料实现了2-氧代-2-芳乙基芳甲酸酯高效合成. 该方法具有底物范围广、原料简单易得、操作简便等优点, 为α-酰氧基羰基衍生物的简捷合成提供了新思路.
成立, 王文蓉, 孙玉倩, 李团结, 于晨侠, 姚昌盛. 氮杂环卡宾(NHC)/银(I)共催化合成2-氧代-2-芳乙基芳甲酸酯[J]. 有机化学, 2021, 41(4): 1607-1613.
Li Cheng, Wenrong Wang, Yuqian Sun, Tuanjie Li, Chenxia Yu, Changsheng Yao. N-Heterocyclic Carbene (NHC)/Ag(I) Co-catalyzed Synthesis of 2-Oxo-2-arylethyl Aryl Formates[J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1607-1613.
| Entry | NHC | Base | Solvent | T/℃ | Yieldb/% |
|---|---|---|---|---|---|
| 1 | 4 | K2CO3 | DCM | 80 | 10 |
| 2 | 5 | K2CO3 | DCM | 80 | 0 |
| 3 | 6 | K2CO3 | DCM | 80 | 45 |
| 4 | 7 | K2CO3 | DCM | 80 | 60 |
| 5 | 7 | K2CO3 | K2CO3 | 80 | 52 |
| 6 | 7 | K2CO3 | K2CO3 | 80 | 15 |
| 7 | 7 | K2CO3 | K2CO3 | 80 | 14 |
| 8 | 7 | K2CO3 | K2CO3 | 80 | 8 |
| 9 | 7 | K3PO4 | DCM | 80 | 52 |
| 10 | 7 | DBU | DCM | 80 | 0 |
| 11 | 7 | DABCO | DCM | 80 | 0 |
| 12 | 7 | Cs2CO3 | DCM | 80 | 10 |
| 13 | 7 | K2CO3 | DCM | 30 | 0 |
| 14 | 7 | K2CO3 | DCM | 40 | 10 |
| 15 | 7 | K2CO3 | DCM | 50 | 47 |
| 16 | 7 | K2CO3 | DCM | 60 | 59 |
| 17 | 7 | K2CO3 | DCM | 70 | 70 |
| Entry | NHC | Base | Solvent | T/℃ | Yieldb/% |
|---|---|---|---|---|---|
| 1 | 4 | K2CO3 | DCM | 80 | 10 |
| 2 | 5 | K2CO3 | DCM | 80 | 0 |
| 3 | 6 | K2CO3 | DCM | 80 | 45 |
| 4 | 7 | K2CO3 | DCM | 80 | 60 |
| 5 | 7 | K2CO3 | K2CO3 | 80 | 52 |
| 6 | 7 | K2CO3 | K2CO3 | 80 | 15 |
| 7 | 7 | K2CO3 | K2CO3 | 80 | 14 |
| 8 | 7 | K2CO3 | K2CO3 | 80 | 8 |
| 9 | 7 | K3PO4 | DCM | 80 | 52 |
| 10 | 7 | DBU | DCM | 80 | 0 |
| 11 | 7 | DABCO | DCM | 80 | 0 |
| 12 | 7 | Cs2CO3 | DCM | 80 | 10 |
| 13 | 7 | K2CO3 | DCM | 30 | 0 |
| 14 | 7 | K2CO3 | DCM | 40 | 10 |
| 15 | 7 | K2CO3 | DCM | 50 | 47 |
| 16 | 7 | K2CO3 | DCM | 60 | 59 |
| 17 | 7 | K2CO3 | DCM | 70 | 70 |
| Entry | R | Ar | Time/h | Product | Yield/% |
|---|---|---|---|---|---|
| 1 | 4-ClC6H4 | C6H5 | 12 | 3a | 70 |
| 2 | 4-BrC6H4 | C6H5 | 12 | 3b | 68 |
| 3 | 4-FC6H4 | C6H5 | 12 | 3c | 58 |
| 4 | 3-BrC6H4 | C6H5 | 12 | 3d | 65 |
| 5 | 3,4-Cl2C6H3 | C6H5 | 12 | 3e | 75 |
| 6 | 4-O2NC6H4 | C6H5 | 12 | 3f | 74 |
| 7 | 4-CH3OC6H4 | C6H5 | 24 | 3g | 48 |
| 8 | Thiophene-2-yl | C6H5 | 12 | 3h | 58 |
| 9 | 3,4-Cl2C6H3 | 4-ClC6H4 | 12 | 3i | 73 |
| 10 | 4-ClC6H4 | 4-ClC6H4 | 12 | 3j | 68 |
| 11 | 4-NO2C6H4 | 4-ClC6H4 | 12 | 3k | 76 |
| 12 | 4-CH3OC6H4 | 4-ClC6H4 | 24 | 3l | 50 |
| 13 | Thiophene-2-yl | 4-ClC6H4 | 12 | 3m | 62 |
| 14 | 3,4-Cl2C6H3 | 4-CH3CH2C6H4 | 12 | 3n | 70 |
| 15 | 4-CH3OC6H4 | 4-CH3CH2C6H4 | 24 | 3o | 45 |
| 16 | Thiophene-2-yl | 4-CH3CH2C6H4 | 12 | 3p | 60 |
| Entry | R | Ar | Time/h | Product | Yield/% |
|---|---|---|---|---|---|
| 1 | 4-ClC6H4 | C6H5 | 12 | 3a | 70 |
| 2 | 4-BrC6H4 | C6H5 | 12 | 3b | 68 |
| 3 | 4-FC6H4 | C6H5 | 12 | 3c | 58 |
| 4 | 3-BrC6H4 | C6H5 | 12 | 3d | 65 |
| 5 | 3,4-Cl2C6H3 | C6H5 | 12 | 3e | 75 |
| 6 | 4-O2NC6H4 | C6H5 | 12 | 3f | 74 |
| 7 | 4-CH3OC6H4 | C6H5 | 24 | 3g | 48 |
| 8 | Thiophene-2-yl | C6H5 | 12 | 3h | 58 |
| 9 | 3,4-Cl2C6H3 | 4-ClC6H4 | 12 | 3i | 73 |
| 10 | 4-ClC6H4 | 4-ClC6H4 | 12 | 3j | 68 |
| 11 | 4-NO2C6H4 | 4-ClC6H4 | 12 | 3k | 76 |
| 12 | 4-CH3OC6H4 | 4-ClC6H4 | 24 | 3l | 50 |
| 13 | Thiophene-2-yl | 4-ClC6H4 | 12 | 3m | 62 |
| 14 | 3,4-Cl2C6H3 | 4-CH3CH2C6H4 | 12 | 3n | 70 |
| 15 | 4-CH3OC6H4 | 4-CH3CH2C6H4 | 24 | 3o | 45 |
| 16 | Thiophene-2-yl | 4-CH3CH2C6H4 | 12 | 3p | 60 |
| [1] |
Lin, L.; Mulholland, N.; Wu, Q.-Y.; Beattie, D.; Huang, S.-W.; Irwin, D.; Clough, J.; Gu, Y.-C.; Yang, G.-F. J. Agric. Food Chem. 2012, 60,4480.
doi: 10.1021/jf300610j pmid: 22439963 |
| [2] |
Wang, X.; Sena Filho, J.G.; Hoover, A.R.; King, J.B.; Ellis, T.K.; Powell, D.R.; Cichewicz, R.H. J. Nat. Prod. 2010, 73,942.
doi: 10.1021/np100142h pmid: 20450206 |
| [3] |
Wang, H.; Wang, Y.; Wang, W.; Fu, P.; Liu, P.; Zhu, W. J. Nat. Prod. 2011, 74,2014.
pmid: 21879714 |
| [4] |
(a) Sabbah, D.A.; Saada, M.; Khalaf, R.A.; Bardaweel, S.; Sweidan, K.; Al-Qirim, T.; Al-Zughier, A.; Halim, H.A.; Sheikh, G.A. Bioorg. Med. Chem. Lett. 2015, 25,3120.
pmid: 23044371 |
|
(b) Che, Y.; Wen, D.; Huang, Z.; Huang, M.; Luo, Y.; Liu, B.; Lu, H.; Wu, Y.; Peng, Y.; Zhang, J. Bioorg. Med. Chem. Lett. 2012, 22,6867.
doi: 10.1016/j.bmcl.2012.09.037 pmid: 23044371 |
|
| [5] |
Dai, L.; Yu, S.; Xiong, W.; Chen, Z.; Xu, T.; Shao, Y.; Chen, J. Adv. Synth. Catal. 2020, 362,1893.
|
| [6] |
Kim, S.H.; Jang, M.; Moon, D.Y.; Park, B.S. Tetrahedron Lett. 2018, 59,4245.
|
| [7] |
Arai, M.A.; Kofuji, Y.; Tanaka, Y.; Yanase, N.; Yamaku, K.; Fuentes, R.G.; Karmakar, U.K.; Ishibashi, M. Org. Biomol. Chem. 2016, 14,3061.
doi: 10.1039/c5ob02537k pmid: 26893289 |
| [8] |
(a) Funk, P.; Motyka, K.; Džubák, P.; Znojek, P.; Gurská, S.; Kusz, J.; McMaster, C.; Hajdúch, M.; Soural, M. RSC Adv. 2015, 5,48861.
|
|
(b) Kadrić, J.; Motyka, K.; Džubák, P.; Hajdúch, M.; Soural, M. Tetrahedron Lett. 2014, 55,3592.
|
|
| [9] |
(a) Prasad, P.K.; Reddi, R.N.; Arumugam, S. Org. Biomol. Chem. 2018, 16,9334.
doi: 10.1039/c8ob02881h pmid: 27827500 |
|
(b) Zhou, X.; Ma, H.; Cao, J.; Liu, X.; Huang, G.; Org. Biomol. Chem. 2016, 14,10070.
pmid: 27827500 |
|
|
(c) Zhu, Y.; Zheng, Y.; Song, W.; Wei, B.; Xuan, L. Tetrahedron Lett. 2018, 59,368.
pmid: 27827500 |
|
|
(d) Zhu, M.; Wei, W.; Yang, D.; Cui, H.; Sun, X.; Wang, H. Org. Biomol. Chem. 2016, 14,10998.
doi: 10.1039/c6ob02173e pmid: 27827500 |
|
| [10] |
(a) Kreibich, M.; Gemander, M.; Peter, D.; Yadav, D.; Koning, C.; Fernandes, M.; Green, I.; van Otterlo, W.; Brückner, R. Eur. J. Org. Chem. 2020, 19,2929.
|
|
(b) Liu, L.; Feng, S.; Li, C. ACS Sustainable Chem. Eng. 2016, 4,6754.
|
|
|
(c) Hu, Y.; Chen, J.; Le, Z.G.; Chen, Z.C.; Zheng, Q.G. Chin. Chem. Lett. 2005, 16,903.
|
|
|
(d) Wang, X.; Li, G.; Yang, Y.; Jiang, J.; Feng, Z.; Zhang, P. Chin. Chem. Lett. 2020, 31,711.
|
|
| [11] |
Mondal, B.; Sahoo, S.C.; Pan, S.C. Eur. J. Org. Chem. 2015, 14,3135.
|
| [12] |
Wang, J.-L.; Wang, J.-Q.; He, L.-N.; Dou, X.-Y.; Wu, F. Green Chem. 2008, 10,1218.
|
| [13] |
Ji, K.; Zhao, Y.; Zhang, L. Angew. Chem. Int. Ed. 2013, 52,6508.
|
| [14] |
Mu, Y.; Chen, Y.; Gao, Y.; Sun, J.; Iqbal, Z.; Wan, Y.; Yang, M.; Yang, Z.; Tang, D. ChemistrySelect 2020, 5,1705.
|
| [15] |
Tian, L.; Guo, Y.; Wei, L.; Wan, J.-P.; Sheng, S. Asian J. Org. Chem. 2019, 8,1484.
|
| [16] |
Li, J.; Yang, Z.; Yang, T.; Yi, J.; Zhou, C. New J. Chem. 2018, 42,1581.
|
| [17] |
Chen, C.; Liu, W.; Zhou, P.; Liu, H. RSC Adv. 2017, 7,20394.
|
| [18] |
Tan, L.; Chen, C.; Liu, W. Beilstein J. Org. Chem. 2017, 13,1079.
pmid: 28684987 |
| [19] |
(a) Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Chem. Rev. 2015, 115,9307.
doi: 10.1021/acs.chemrev.5b00060 pmid: 23225526 |
|
(b) Enders, D.; Niemeier, O.; Henseler, A. Chem. Rev. 2007, 107,5606.
doi: 10.1021/cr068372z pmid: 23225526 |
|
|
(c) Wang, Z.; Li, R.; Qian, H.; Yao, C. Chin. J. Org. Chem. 2019, 39,2075. (in Chinese)
pmid: 23225526 |
|
|
( 王占林, 李如一, 钱辉旻, 姚昌盛, 有机化学, 2019, 39,2075.)
pmid: 23225526 |
|
|
(d) Yao, C.; Xiao, Z.; Liu, R.; Li, T.; Jiao, W.; Yu, C. Chem. Eur. J. 2013, 19,456.
pmid: 23225526 |
|
|
(e) Li, S.; Yang, W.; Luo, X.; Yao, C. Chin. J. Org. Chem. 2019, 39,1404. (in Chinese)
pmid: 23225526 |
|
|
( 李莎, 杨雯涵, 罗鲜, 姚昌盛, 有机化学, 2019, 39,1404.)
pmid: 23225526 |
|
|
(f) Li, S.; Xu, J.; Luo, X.; Yang, W.; Yao, C. Chin. J. Org. Chem. 2020, 40,470. (in Chinese)
pmid: 23225526 |
|
|
( 李莎, 徐嘉煜, 罗鲜, 杨雯涵, 姚昌盛, 有机化学, 2020, 40,470.)
pmid: 23225526 |
|
|
(g) Zhang, Y.; Xing, F.; Feng, Z.; Du, G.; Gu, C.; He, L. Chin. J. Org. Chem. 2020, 40,1608. (in Chinese)
pmid: 23225526 |
|
|
( 张阳, 邢芬, 冯泽男, 杜广芬, 顾承志, 何林, 有机化学, 2020, 40,1608.)
pmid: 23225526 |
|
|
(h) Wang, A.; Xiao, Y.; Zhou, Y.; Xu, J.; Liu, H. Chin. J. Org. Chem. 2017, 37,2590. (in Chinese)
pmid: 23225526 |
|
|
( 王翱, 肖永龙, 周宇, 徐进宜, 柳红, 有机化学, 2017, 37,2590.)
pmid: 23225526 |
|
|
(i) Qu, M.; He, J. Chin. J. Org. Chem. 2011, 31,1388. (in Chinese)
pmid: 23225526 |
|
|
( 屈孟男, 何金梅, 有机化学, 2011, 31,1388.)
pmid: 23225526 |
|
| [20] |
Reddi, R.N.; Malekar, P.V.; Sudalai, A. Org. Biomol. Chem. 2013, 11,6477.
doi: 10.1039/c3ob41551a pmid: 23969565 |
| [21] |
Reddi, R.N.; Gontala, A.; Prasad, P.K.; Sudalai, A. Asian J. Org. Chem. 2016, 5,48.
|
| [22] |
Forte, G.; Chiarotto, I.; Inesi, A.; Loreto, M.A.; Feroci, M. Adv. Synth. Catal. 2014, 356,1773.
|
| [23] |
Lu, H.; Liu, J.-Y.; Li, H.-Y.; Xu, P.-F. Acta Chim. Sinica 2018, 76,831. (in Chinese)
|
|
( 鲁鸿, 刘金宇, 李红玉, 许鹏飞, 化学学报, 2018, 76,831.)
|
|
| [24] |
Nemoto, T.; Fukuda, T.; Hamada, Y. Tetrahedron Lett. 2006, 47,4365.
|
| [25] |
(a) Namitharan, K.; Zhu, T.; Cheng, J.; Zheng, P.; Li, X.; Yang, S.; Song, B.-A.; Chi, Y.R. Nat. Commun. 2014, 5,3982.
doi: 10.1038/ncomms4982 pmid: 24865392 |
|
(b) Chen, J.; Yuan, P.; Wang, L.; Huang, Y. J. Am. Chem. Soc. 2017, 139,7045.
pmid: 24865392 |
|
| [26] |
DiRocco, D.A.; Rovis, T. J. Am. Chem. Soc. 2012, 134,8094.
pmid: 22548244 |
| [27] |
Chen, Z.-W.; Ye, D.-N.; Ye, M.; Zhou, Z.-G.; Li, S.-H.; Liu, L.-X. Tetrahedron Lett. 2014, 55,1373.
|
| [28] |
Jia, Y.; Li, T.; Yu, C.; Jiang, B.; Yao, C. Org. Biomol. Chem. 2016, 14,1982.
doi: 10.1039/c5ob02336j pmid: 26754554 |
| [29] |
(a) Liu, Y.K.; Li, R.; Yue, L.; Li, B.J.; Chen, Y.C.; Wu, Y.; Ding, L.S. Org. Lett. 2006, 8,1521.
pmid: 16597100 |
|
(b) Xia, Z.-H.; Dai, L.; Gao, Z.-H.; Ye, S. Chem. Commun. 2020, 56,1525.
pmid: 16597100 |
|
|
(c) Gao, Z.-H.; Xia, Z.-H.; Dai, L.; Ye, S. Adv. Synth. Catal. 2020, 362,1819.
pmid: 16597100 |
|
| [30] |
Liao, L.; Zhang, H.; Zhao, X. ACS Catal. 2018, 8,6745.
|
| [31] |
Hu, Y.; Chen, J.; Le, Z.G.; Chen, Z.C.; Zheng, Q.G. Chin. Chem. Lett. 2005, 16,903.
|
| [1] | 李莎, 徐嘉煜, 罗鲜, 杨雯涵, 姚昌盛. 氮杂环卡宾催化下官能化萘并吡喃酮的串联合成[J]. 有机化学, 2020, 40(2): 470-477. |
| [2] | 王占林, 李如一, 钱辉旻, 姚昌盛. 温度对氮杂环卡宾催化下α-溴代烯醛与烯胺酮的[3+3]环化反应影响[J]. 有机化学, 2019, 39(7): 2075-2083. |
| [3] | 李莎, 杨雯涵, 罗鲜, 姚昌盛. 氮杂环卡宾(NHC)催化下多取代环戊烯的串联合成[J]. 有机化学, 2019, 39(5): 1404-1410. |
| [4] | 和振秀, 周永云, 孙蔚青, 樊瑞峰, 沈国礼, 樊保敏. 铑/锌共催化苯酚对氧杂苯并降冰片烯的不对称开环反应研究[J]. 有机化学, 2019, 39(3): 754-760. |
| [5] | 王华斌, 付强, 张智杰, 高明, 姬建新, 易东. 盐酸促进、铜/铁共催化2-取代丙烯酸酯与膦氧类化合物的脱酯氧膦化反应[J]. 有机化学, 2018, 38(8): 1977-1984. |
| [6] | 王超, 邓楠, 王玲玲, 许定健, 姚小泉. 铜-银双金属纳米颗粒催化的醛-端炔加成反应研究[J]. 有机化学, 2016, 36(5): 1034-1043. |
| [7] | 孙哲, 何金梅, 屈孟男, 李侃社. 共催化在有机合成反应中的研究进展[J]. 有机化学, 2015, 35(6): 1250-1259. |
| [8] | 倪懿, 郭鑫, 胡文浩, 刘顺英. 基于醋酸铑、磺酸和手性亚磺酰胺基脲共催化的α-重氮酯与酰胺不对称N—H插入反应研究[J]. 有机化学, 2014, 34(1): 107-111. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||