有机化学 ›› 2021, Vol. 41 ›› Issue (7): 2885-2890.DOI: 10.6023/cjoc202102034 上一篇 下一篇
研究论文
收稿日期:2021-02-20
修回日期:2021-03-04
发布日期:2021-03-25
通讯作者:
徐亮
基金资助:
Yuanzhi Li, Mengqian Zhu, Liang Xu( )
)
Received:2021-02-20
Revised:2021-03-04
Published:2021-03-25
Contact:
Liang Xu
Supported by:文章分享
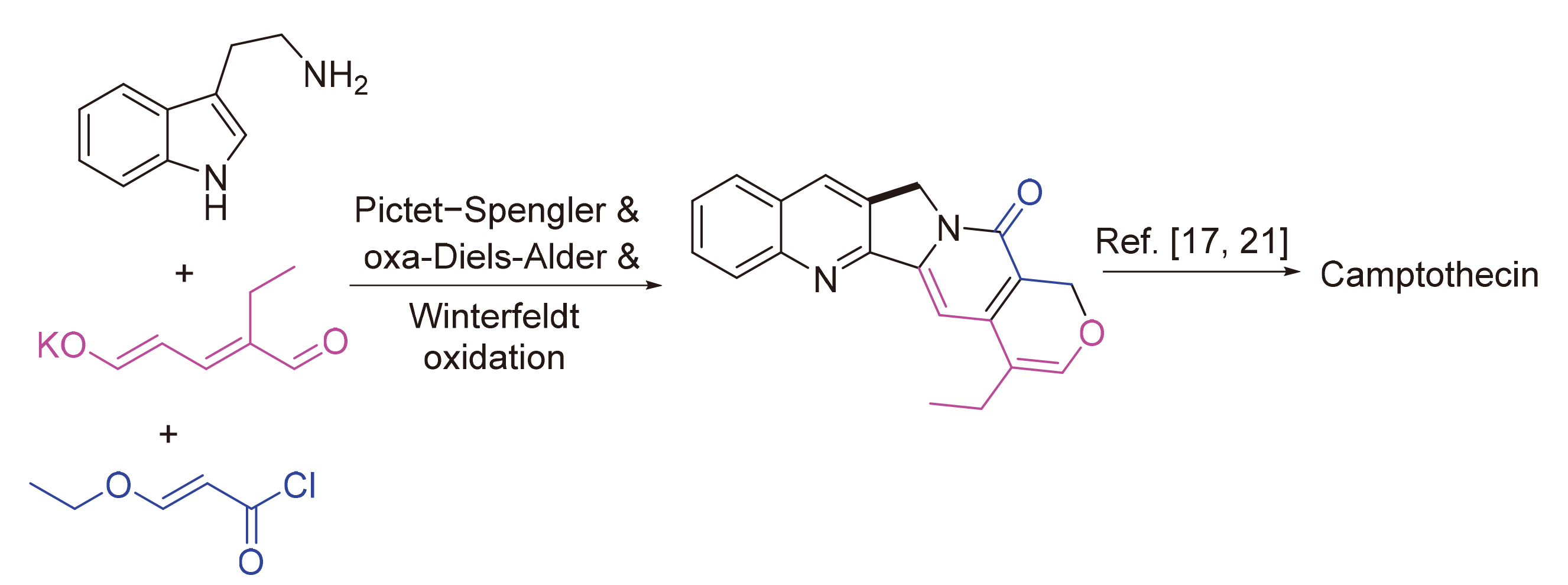
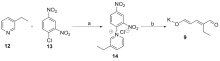
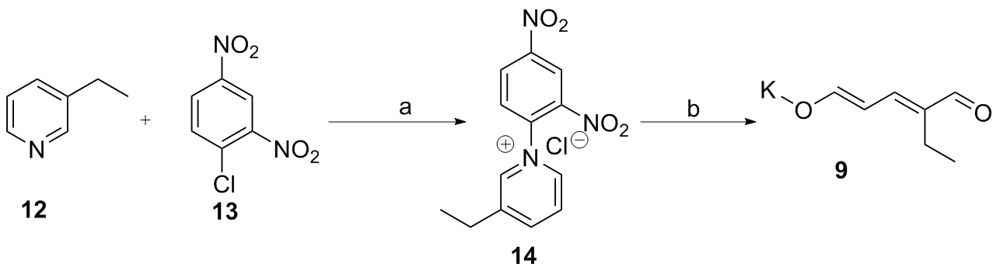
以色胺和方便易得的乙基戊烯二醛盐为起始物, 以简洁的步骤(最终实现喜树碱全合成共9步反应)完成了喜树碱的生源启发下的无保护基形式合成. 合成路线涉及关键的Pictet-Spengler反应, 分子内氧杂Diels-Alder反应高效构建单萜吲哚中间体, 以及Winterfeldt高效仿生氧化吲哚合成喹啉酮结构.
李远志, 朱孟倩, 徐亮. 生源启发下的喜树碱的简洁形式合成[J]. 有机化学, 2021, 41(7): 2885-2890.
Yuanzhi Li, Mengqian Zhu, Liang Xu. A Concise Biogenetically Inspired Formal Synthesis of Camptothecin[J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2885-2890.


| [1] |
Wall,M. E.; Wani,M. C.; Cook,C. E.; Palmer,K. H.; McPhail,A. T.; Sim,G. A. J. Am. Chem. Soc. 1966, 88,3888.
doi: 10.1021/ja00968a057 |
| [2] |
Moertel,C. G.; Schutt,A. J.; Reitemeier,R. J.; Hahn,R. G. Cancer Chemother. Rep. 1972, 56,95.
|
| [3] |
(a) Hsiang,Y. H.; Hertzberg, R.; Hecht, S.; Liu,L. F. J. Biol. Chem. 1985, 260,14873.
doi: 10.1016/S0021-9258(17)38654-4 |
|
(b) Hertzberg,R. P.; Busby,R. W.; Caranfa,M. J.; Holden,K. G.; John-son,R. K.; Hecht,S. M.; Kingsbury,W. D. J. Biol. Chem. 1990, 265,19287.
doi: 10.1016/S0021-9258(17)30656-7 |
|
|
(c) Pommier, Y.; Kohlhagen, G.; Kohn,K. W.; Leteurtre, F.; Wani,M. C.; Wall,M. E. Proc. Natl. Acad. Sci. U. S. A. 1995, 92,8861.
doi: 10.1073/pnas.92.19.8861 |
|
| [4] |
(a) Uehling,D. E.; Nanthakumar,S. S.; Croom, D.; Emerson,D. L.; Leitner,P. P.; Luzzio,M. J.; McIntyre, G.; Morton, B.; Profeta, S. J. Med. Chem. 1995, 38,1106.
doi: 10.1021/jm00007a008 |
|
(b) Jew,S. S.; Kim,H. J.; Kim,M. G.; Roh,E. Y.; Cho,Y. S.; Kim,J. K.; Cha,K. H.; Lee,K. K.; Han,H. J.; Choi,J. Y.; Lee, H. Bioorg. Med. Chem. Lett. 1996, 6,845.
doi: 10.1016/0960-894X(96)00131-X |
|
|
(c) Pan,X. D.; Han, R.; Sun,P. Y. Bioorg. Med. Chem. Lett. 2003, 13,3739.
doi: 10.1016/j.bmcl.2003.08.012 |
|
|
(d) Tangirala,R. S.; Dixon, R.; Yang,D. Z.; Ambrus, A.; Antony, S.; Agama, K.; Pommier, Y.; Curran,D. P. Bioorg. Med. Chem. Lett. 2005, 15,4736.
doi: 10.1016/j.bmcl.2005.07.074 |
|
|
(e) Hutchinson,C. R. Tetrahedron 1981, 37,1047.
doi: 10.1016/S0040-4020(01)92034-4 |
|
|
(f) Du, W. Tetrahedron 2003, 59,8649.
doi: 10.1016/S0040-4020(03)01203-1 |
|
|
(g) Thomas,C. J.; Rahier,N. J.; Hecht,S. M. Bioorg. Med. Chem. 2004, 12,1585.
doi: 10.1016/j.bmc.2003.11.036 |
|
|
(h) Li,Q. Y.; Zu,Y. G.; Shi,R. Z.; Yao,L. P. Curr. Med. Chem. 2006, 13,2021.
doi: 10.2174/092986706777585004 |
|
|
(i) Verma,R. P.; Hansch, C. Chem. Rev. 2009, 109,213.
doi: 10.1021/cr0780210 |
|
|
(j) Martino, E.; Volpe,S. D.; Terribile, E.; Benetti, E.; Sakaj, M.; Centamore, A.; Sala, A.; Collina, S. Bioorg. Med. Chem. Lett. 2017, 27,701.
doi: 10.1016/j.bmcl.2016.12.085 |
|
|
(k) Pan, P.; Chen, J.; Li,M. Y.; Yu,H. D.; Zhao,J. J.; Ni, J.; Wang,X. W.; Sun,H. Y.; Tian, S.; Zhu, F.; Liu, F.; Huang, Y.; Hou,T. J. J. Med. Chem. 2018, 61,8613.
doi: 10.1021/acs.jmedchem.8b00498 |
|
| [5] |
Kawato, Y.; Aonuma, M.; Hirota, Y.; Kuga, H.; Sato, K. Cancer Res. 1991, 51,4187.
|
| [6] |
Kingsbury,W. D.; Boehm,J. C.; Jakas,D. R.; Holden,K. G.; Hecht,S. M.; Gallagher, G.; Caranfa,M. J.; Mccabe,F. L.; Faucette,L. F.; Johnson,R. K.; Hertzberg,R. P. J. Med. Chem. 1991, 34,98.
doi: 10.1021/jm00105a017 |
| [7] |
Ban,H. J.; Oh,I. J.; Kim,K. S.; Ju,J. Y.; Kwon,Y. S.; Kim,Y. I.; Lim,S. C.; Kim,Y. C. Tuberc. Respir. Dis. 2009, 66,93.
doi: 10.4046/trd.2009.66.2.93 |
| [8] |
Stork, G.; Schultz,A. G. J. Am. Chem. Soc. 1971, 93,4074.
doi: 10.1021/ja00745a056 |
| [9] |
For reviews, see: (a) Schultz,A. G. Chem. Rev. 1973, 73,385.
doi: 10.1021/cr60284a004 |
|
(b) Shamma, M.; Georgiev,V. St. J. Pharm. Sci. 1974, 63,163.
doi: 10.1002/jps.2600630203 |
|
|
(c) Takayama, H.; Kitajima, M.; Aimi, N. J. Synth. Org. Chem. 1999, 57,181.
doi: 10.5059/yukigoseikyokaishi.57.181 |
|
|
(d) Baurle, S.; Koert, U. In Organic Synthesis Highlights IV, Ed.: Schmalz, H. G., Wiley-VCH, Weinheim, 2000, p. 232.
|
|
|
(e) Kawato, Y.; Terasawa, H. Prog. Med. Chem. 1997, 34,69.
|
|
|
(f) Hutchinson,C. R. Tetrahedron 1981, 37,1047.
doi: 10.1016/S0040-4020(01)92034-4 |
|
|
(g) Thomas,C. J.; Rahier,N. J.; Hecht,S. M. Bioorg. Med. Chem. 2004, 12,1585.
doi: 10.1016/j.bmc.2003.11.036 |
|
|
(h) Li,Q. Y.; Zu,Y. G.; Yao,L. P. Curr. Med. Chem. 2006, 13,2021.
doi: 10.2174/092986706777585004 |
|
|
(i) Liew,S. T.; Yang,L. X. Curr. Pharm. Des. 2008, 14,1078.
doi: 10.2174/138161208784246180 |
|
|
(j) Du, W. Tetrahedron 2003, 59,8649.
doi: 10.1016/S0040-4020(03)01203-1 |
|
|
(k) Chen, L.; Chen,F. E. Synlett 2017, 28,1134.
doi: 10.1055/s-0036-1588738 |
|
|
For recent research articles after 2017, see: (l) Wang,X. L.; Xu,L. J.; Xiong,F. J.; Wu, Y.; Chen,F. E. Tetrahedron: Asymmetry 2017, 28,843.
doi: 10.1016/j.tetasy.2017.04.013 |
|
|
(m) Yuan, Y.; Dong,W. H.; Gao,X. S.; Xie,X. M.; Curran,D. P.; Zhang,Z. G. Chin. J. Chem. 2018, 36,1035.
doi: 10.1002/cjoc.201800358 |
|
|
(n) Liu, Q.; Huang,G. X.; Liu,M. J.; Chen,F. E. Eur. J. Org. Chem. 2019,6024.
|
|
|
(o) Liu, Q.; Huang,G. X.; Liu,M. J.; Chen,F. E. Synthesis 2019, 51,3506.
doi: 10.1055/s-0037-1611870 |
|
|
(p) Liu, Q.; Liu,M. J.; Huang,G. X.; Chen,F. E. Tetrahedron 2019, 75,2647.
doi: 10.1016/j.tet.2019.03.028 |
|
|
(q) Dong,W. H.; Yuan, Y.; Hu, B.; Gao,X. S.; Gao, H.; Xie,X. M.; Zhang,Z. G. Org. Lett. 2018, 20,80.
doi: 10.1021/acs.orglett.7b03395 |
|
| [10] |
(a) Gaich, T.; Baran,P. S. J. Org. Chem. 2010, 75,4657.
doi: 10.1021/jo1006812 |
|
(b) Zheng, K.; Shen,D. F.; Zhang,B. B.; Hong, R. J. Org. Chem. 2020, 85,13818.
doi: 10.1021/acs.joc.0c01930 |
|
|
(c) Vieira de Castro, T.; Yahiaoui, O.; Peralta, R.A.; Fallon, T.; Lee, V.; George, J.H. Org. Lett. 2020, 22,8161.
doi: 10.1021/acs.orglett.0c03156 |
|
| [11] |
Wenkert, E.; Dave,K. G.; Lewis,R. G.; Sprague,P. W. J. Am. Chem. Soc. 1967, 89,6741.
doi: 10.1021/ja01001a061 |
| [12] |
(a) Winterfeldt, E.; Rodunz, H. Chem. Commun. 1971,374.
|
|
(b) Winterfeldt, E.; Korth, T.; Pike, D.; Boch, M. Angew. Chem.,Int. Ed. 1972, 11,289.
|
|
| [13] |
(a) Brown,R. T.; Leonard, J.; Sleigh,S. K. Phytochemistry 1978, 17,899.
doi: 10.1016/S0031-9422(00)88642-2 |
|
(b) Brown,R. T.; Liu, J.; Santos,C. A.M., Tetrahedron Lett. 2000, 41,859.
doi: 10.1016/S0040-4039(99)02210-8 |
|
| [14] |
Nguyen,T. M.; Peixoto, S.; Ouairy, C.; Nguyen,T. D.; Bénéchie, M.; Marazano, C.; Michel, P. Synthesis 2010,103.
|
| [15] |
Yan,L. H.; Skiredj, A.; Dory, Y.; Delpech, B.; Poupon, E. Eur. J. Org. Chem. 2014,4973.
|
| [16] |
(a) Nuhant, P.; Raikar,S. B.; Wypych,J. C.; Delpech, B.; Mazarano, C. J. Org. Chem. 2009, 74,9413.
doi: 10.1021/jo9019545 |
|
(b) Overman,L. E.; Robichaud,A. J. J. Am. Chem. Soc. 1989, 111,300.
doi: 10.1021/ja00183a046 |
|
| [17] |
Liu,G. S.; Dong,Q. L.; Yao,Y. S.; Yao,Z. J. Org. Lett. 2008, 10,5393.
doi: 10.1021/ol802250y |
| [18] |
Yuan,Y. H.; Han, X.; Zhu,F. P.; Tian,J. M.; Zhang,F. M.; Zhang,X. M.; Tu,Y. Q.; Wang,S. H.; Guo, X. Nat. Commun. 2019, 10,3394.
doi: 10.1038/s41467-019-11382-8 |
| [19] |
Takayama, H.; Ishikawa, H.; Kurihara, M.; Kitajima, M.; Aimi, N.; Ponglux, D.; Koyama, F.; Matsumoto, K.; Moriyama, T.; Yamamoto,L. T.; Watanabe, K.; Murayama, T.; Horie, S. J. Med. Chem. 2002, 45,1949.
doi: 10.1021/jm010576e |
| [20] |
Thomas,O. P.; Zaparucha, A.; Husson,H. P. Eur. J. Org. Chem. 2002,157.
|
| [21] |
Li, K.; Ou,J. J.; Gao,S. H. Angew. Chem., nt. Ed. 2016, 55,14778.
|
| [1] | 张晓雨, 李欣燕, 崔冰, 邵志晖, 赵铭钦. 四氢-β-咔啉衍生物的设计、合成及抗氧化性能研究[J]. 有机化学, 2023, 43(8): 2885-2894. |
| [2] | 张世举, 李晓彤, 王燕, 郑宇璁, 韩世清, 郁惠蕾, 黄莎华. 抗革兰氏阴性菌耐格霉素的形式合成[J]. 有机化学, 2020, 40(2): 521-527. |
| [3] | 闫萌, 彭文昶, 王辉, 张丹维, 黎占亭. 超分子有机框架对喜树碱类开环羧酸盐的负载及其内酯化动力学[J]. 有机化学, 2019, 39(9): 2567-2573. |
| [4] | 徐姣, 张丽宏, 张美琦, 刘秀波, 马伟, 唐益鑫, 王道林. 碘-二甲基亚砜促进新型四环噻唑并[3',2':2,3]吡啶并[4,5-d]吡啶并[1,2-a]嘧啶酮类衍生物的合成[J]. 有机化学, 2019, 39(10): 2808-2812. |
| [5] | 徐姣, 马玲, 刘秀波, 马伟, 马岩, 王道林. 新型苯并噻吩稠合吡啶并[1,2-a]嘧啶衍生物的合成及其抑菌活性[J]. 有机化学, 2018, 38(7): 1680-1686. |
| [6] | 王冬, 王道林, 钱建华. 苯并噻吩并[3',2':2,3]吡啶并[4,5-d]噻唑并[3,2-a]嘧啶酮类衍生物的有效合成[J]. 有机化学, 2017, 37(3): 698-703. |
| [7] | 孟光荣, 李嘉俊, 王国林, 董孟杰, 张倩. 10-羟基喜树碱氨基酸缀合物的合成及抗肿瘤活性研究[J]. 有机化学, 2014, 34(1): 155-160. |
| [8] | 孟光荣, 李嘉俊, 张倩, 马红梅. 微波技术在10-羟基喜树碱醚化反应中的应用[J]. 有机化学, 2012, 32(12): 2378-2381. |
| [9] | 肖锋, 罗宇, 吕伟, 汤杰. 吉咪替康的合成新方法[J]. 有机化学, 2010, 30(02): 311-313. |
| [10] | 段华鑫a ; 张殊佳*,a,b ; 周 鹏a. 20(S)-O-喜树碱肉桂酸酯衍生物的合成及其抗肿瘤活性研究[J]. 有机化学, 2009, 29(05): 724-729. |
| [11] | 缪震元,张万年,姚建忠,盛春泉,徐辉张珉,张晶,游亮,车晓颖. 10-酯基高喜树碱的全合成及抗肿瘤活性研究[J]. 有机化学, 2006, 26(9): 1221-1224. |
| [12] | 赵宝祥, 沙磊, 谭伟, 左华, 王大威. 通过氧杂Pictet-Spengler反应从1-烷(苯)氧基-3-(3,4-亚甲基二氧)苯基-2-丙醇合成异色满衍生物[J]. 有机化学, 2004, 24(10): 1300-1303. |
| [13] | 刘建利. 喜树碱的仿生合成:一个四分之一世纪的故事[J]. 有机化学, 2003, 23(5): 432-437. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||