有机化学 ›› 2022, Vol. 42 ›› Issue (7): 2124-2133.DOI: 10.6023/cjoc202202006 上一篇 下一篇
所属专题: 有机氟化学虚拟合辑
研究论文
李响a,b, 张依凡a, 陆凯琳b, 刘石惠a,*( ), 张永强b,*(
), 张永强b,*( )
)
收稿日期:2022-02-06
修回日期:2022-03-26
发布日期:2022-08-09
通讯作者:
刘石惠, 张永强
作者简介:基金资助:
Xiang Lia,b, Yifan Zhanga, Kailin Lub, Shihui Liua( ), Yongqiang Zhangb(
), Yongqiang Zhangb( )
)
Received:2022-02-06
Revised:2022-03-26
Published:2022-08-09
Contact:
Shihui Liu, Yongqiang Zhang
About author:Supported by:文章分享
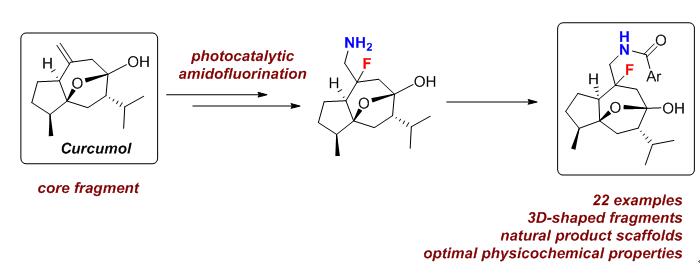
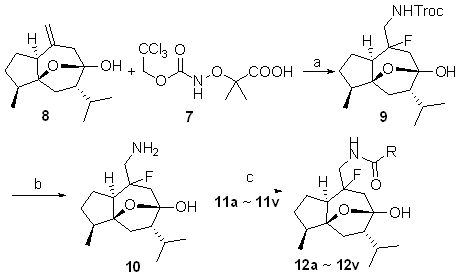
富含sp3中心的三维天然产物结构由于其独特的生物活性, 在药物化学及生物化学方面越来越受到重视. 以天然产物莪术醇为基础, 通过光催化的胺氟化反应, 高效引入胺和氟两个潜在的结合或反应位点, 构建了以三维形状天然产物为核心的片段库. 通过这一策略, 合成了包含24个新型莪术醇衍生物的片段库, 进一步计算结果表明, 所获得的片段库具有高三维度、天然产物相似性和优质的片段属性, 在基于片段的药物发现中开辟了一个新的化学空间.
李响, 张依凡, 陆凯琳, 刘石惠, 张永强. 基于莪术醇胺氟化结构修饰的三维天然产物片段库的构建[J]. 有机化学, 2022, 42(7): 2124-2133.
Xiang Li, Yifan Zhang, Kailin Lu, Shihui Liu, Yongqiang Zhang. Aminofluorination-Based Structural Modification of Curcumol for the Construction of 3D-Shaped Natural Product Fragment Library[J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2124-2133.
| Property | Ideal Rangeb | Curcumol |
|---|---|---|
| MW | ≤300 | 236.35 |
| Alog P | 0~2 | 2.63 |
| Heavy atom count | 10-16 | 17 |
| Hydrogen-bond acceptors | ≤3 | 2 |
| Hydrogen-bond donors | ≤3 | 1 |
| Rotatable bond count | ≤3 | 1 |
| Carbon sp3 fraction | — | 0.866667 |
| Plane of best fit/nm | — | 0.1003 |
| Polar surface area/nm2 | ≤0.60 | 0.2946 |
| Property | Ideal Rangeb | Curcumol |
|---|---|---|
| MW | ≤300 | 236.35 |
| Alog P | 0~2 | 2.63 |
| Heavy atom count | 10-16 | 17 |
| Hydrogen-bond acceptors | ≤3 | 2 |
| Hydrogen-bond donors | ≤3 | 1 |
| Rotatable bond count | ≤3 | 1 |
| Carbon sp3 fraction | — | 0.866667 |
| Plane of best fit/nm | — | 0.1003 |
| Polar surface area/nm2 | ≤0.60 | 0.2946 |

| Compd. | Mr | Alog P | Carbon sp3 fraction | Plane of Best Fit/nm | Polar Surface Area/nm2 |
|---|---|---|---|---|---|
| 9 | 446.769 | 3.84 | 0.944 | 0.0989 | 0.67.79 |
| 10 | 271.371 | 1.70 | 1.000 | 0.0993 | 0.5548 |
| 12a | 375.477 | 3.33 | 0.682 | 0.1056 | 0.5856 |
| 12b | 389.503 | 3.35 | 0.696 | 0.0851 | 0.5856 |
| 12c | 389.503 | 3.33 | 0.696 | 0.0979 | 0.5856 |
| 12d | 389.503 | 3.34 | 0.696 | 0.1233 | 0.5856 |
| 12e | 405.503 | 3.24 | 0.696 | 0.1254 | 0.67.79 |
| 12f | 431.583 | 4.76 | 0.731 | 0.1071 | 0.5856 |
| 12g | 393.467 | 3.25 | 0.682 | 0.0925 | 0.5856 |
| 12h | 409.922 | 3.92 | 0.682 | 0.0931 | 0.5856 |
| 12i | 454.373 | 3.89 | 0.682 | 0.1061 | 0.5856 |
| 12j | 433.513 | 3.28 | 0.667 | 0.1294 | 0.8486 |
| 12k | 400.486 | 3.02 | 0.652 | 0.1042 | 0.8235 |
| 12l | 389.503 | 3.27 | 0.696 | 0.0940 | 0.5856 |
| 12m | 401.514 | 3.75 | 0.625 | 0.1048 | 0.5856 |
| 12n | 403.530 | 3.63 | 0.708 | 0.1356 | 0.5856 |
| 12o | 435.529 | 3.33 | 0.708 | 0.1087 | 0.7702 |
| 12p | 425.536 | 4.22 | 0.577 | 0.1168 | 0.5856 |
| 12q | 365.439 | 2.77 | 0.750 | 0.0997 | 0.7170 |
| 12r | 415.950 | 3.85 | 0.750 | 0.1076 | 0.5856 |
| 12s | 455.361 | 3.03 | 0.714 | 0.1166 | 0.7145 |
| 12t | 377.453 | 1.50 | 0.750 | 0.1005 | 0.8434 |
| 12u | 425.557 | 2.52 | 0.727 | 0.1176 | 0.7563 |
| 12v | 490.427 | 3.00 | 0.714 | 0.1071 | 0.7563 |
| Average | 407.366 | 3.30 | 0.718 | 0.1074 | 0.6564 |
| Compd. | Mr | Alog P | Carbon sp3 fraction | Plane of Best Fit/nm | Polar Surface Area/nm2 |
|---|---|---|---|---|---|
| 9 | 446.769 | 3.84 | 0.944 | 0.0989 | 0.67.79 |
| 10 | 271.371 | 1.70 | 1.000 | 0.0993 | 0.5548 |
| 12a | 375.477 | 3.33 | 0.682 | 0.1056 | 0.5856 |
| 12b | 389.503 | 3.35 | 0.696 | 0.0851 | 0.5856 |
| 12c | 389.503 | 3.33 | 0.696 | 0.0979 | 0.5856 |
| 12d | 389.503 | 3.34 | 0.696 | 0.1233 | 0.5856 |
| 12e | 405.503 | 3.24 | 0.696 | 0.1254 | 0.67.79 |
| 12f | 431.583 | 4.76 | 0.731 | 0.1071 | 0.5856 |
| 12g | 393.467 | 3.25 | 0.682 | 0.0925 | 0.5856 |
| 12h | 409.922 | 3.92 | 0.682 | 0.0931 | 0.5856 |
| 12i | 454.373 | 3.89 | 0.682 | 0.1061 | 0.5856 |
| 12j | 433.513 | 3.28 | 0.667 | 0.1294 | 0.8486 |
| 12k | 400.486 | 3.02 | 0.652 | 0.1042 | 0.8235 |
| 12l | 389.503 | 3.27 | 0.696 | 0.0940 | 0.5856 |
| 12m | 401.514 | 3.75 | 0.625 | 0.1048 | 0.5856 |
| 12n | 403.530 | 3.63 | 0.708 | 0.1356 | 0.5856 |
| 12o | 435.529 | 3.33 | 0.708 | 0.1087 | 0.7702 |
| 12p | 425.536 | 4.22 | 0.577 | 0.1168 | 0.5856 |
| 12q | 365.439 | 2.77 | 0.750 | 0.0997 | 0.7170 |
| 12r | 415.950 | 3.85 | 0.750 | 0.1076 | 0.5856 |
| 12s | 455.361 | 3.03 | 0.714 | 0.1166 | 0.7145 |
| 12t | 377.453 | 1.50 | 0.750 | 0.1005 | 0.8434 |
| 12u | 425.557 | 2.52 | 0.727 | 0.1176 | 0.7563 |
| 12v | 490.427 | 3.00 | 0.714 | 0.1071 | 0.7563 |
| Average | 407.366 | 3.30 | 0.718 | 0.1074 | 0.6564 |
| [1] |
(a) Jencks, W. P. Proc. Natl. Acad. Sci. 1981, 78, 4046.
doi: 10.1073/pnas.78.7.4046 pmid: 21695633 |
|
(b) Fattori, D. Drug Discovery Today 2004, 9, 229.
doi: 10.1016/S1359-6446(03)03007-1 pmid: 21695633 |
|
|
(c) Congreve, M.; Chessari, G.; Tisi, D.; Woodhead, A. J. J. Med. Chem. 2008, 51, 3661.
doi: 10.1021/jm8000373 pmid: 21695633 |
|
|
(d) Murray, C. W.; Rees, D. C. Nat. Chem. 2009, 1, 187.
doi: 10.1038/nchem.217 pmid: 21695633 |
|
|
(e) Scott, D. E.; Coyne, A. G.; Hudson, S. A.; Abell, C. Biochemistry 2012, 51, 4990.
doi: 10.1021/bi3005126 pmid: 21695633 |
|
|
(f) Erlanson, D. A. Top. Curr. Chem. 2012, 317, 1.
doi: 10.1007/128_2011_180 pmid: 21695633 |
|
|
(g) Baker, M. Nat. Rev. Drug Discovery 2013, 12, 5.
doi: 10.1038/nrd3926 pmid: 21695633 |
|
|
(h) Erlanson, D. A.; Fesik, S. W.; Hubbard, R. E.; Jahnke, W.; Jhoti, H. Nat. Rev. Drug Discovery 2016, 15, 605.
doi: 10.1038/nrd.2016.109 pmid: 21695633 |
|
|
(i) Bancet, A.; Raingeval, C.; Lomberget, T.; Le Borgne, M.; Guichou, J.-F.; Krimm, I. J. Med. Chem. 2020, 63, 11420.
doi: 10.1021/acs.jmedchem.0c00242 pmid: 21695633 |
|
|
(j) Vaidergorn, M. M.; da Silva Emery, F.; Ganesan, A. J. Med. Chem. 2021, 64, 13980.
doi: 10.1021/acs.jmedchem.1c00787 pmid: 21695633 |
|
| [2] |
(a) Lovering, F.; Bikker, J.; Humblet, C. J. Med. Chem. 2009, 52, 6752.
doi: 10.1021/jm901241e pmid: 19827778 |
|
(b) Meanwell, N. A. Chem. Res. Toxicol. 2011, 24, 1420.
doi: 10.1021/tx200211v pmid: 19827778 |
|
|
(c) Shultz, M. D. J. Med. Chem. 2019, 62, 1701.
doi: 10.1021/acs.jmedchem.8b00686 pmid: 19827778 |
|
| [3] |
(a) Morley, A. D.; Pugliese, A.; Birchall, K.; Bower, J.; Brennan, P.; Brown, N.; Chapman, T.; Drysdale, M.; Gilbert, I. H.; Hoelder, S.; Jordan, A.; Ley, S. V.; Merritt, A.; Miller, D.; Swarbrick, M. E.; Wyatt, P. G. Drug Discov. Today 2013, 18, 1221.
doi: 10.1016/j.drudis.2013.07.011 |
|
(b) Chen, H.; Zhou, X.; Wang, A.; Zheng, Y.; Gao, Y.; Zhou, J. Drug Discov. Today 2015, 20, 105.
doi: 10.1016/j.drudis.2014.09.015 |
|
| [4] |
(a) Hung, A. W.; Ramek, A.; Wang, Y.; Kaya, T.; Wilson, J. A.; Clemons, P. A.; Young, D. W. Proc. Natl. Acad. Sci. 2011, 108, 6799.
doi: 10.1073/pnas.1015271108 pmid: 33892142 |
|
(b) Keserű, G. M.; Erlanson, D. A.; Ferenczy, G. G.; Hann, M. M.; Murray, C. W.; Pickett, S. D. J. Med. Chem. 2016, 59, 8189.
doi: 10.1021/acs.jmedchem.6b00197 pmid: 33892142 |
|
|
(c) Murray, C. W.; Rees, D. C. Angew. Chem. Int. Ed. 2016, 55, 488.
doi: 10.1002/anie.201506783 pmid: 33892142 |
|
|
(d) Kidd, S. L.; Osberger, T. J.; Mateu, N.; Sore, H. F.; Spring, D. R. Front. Chem. 2018, 6, 460.
doi: 10.3389/fchem.2018.00460 pmid: 33892142 |
|
|
(e) Talele, T. T. J. Med. Chem. 2020, 63, 13291.
doi: 10.1021/acs.jmedchem.0c00829 pmid: 33892142 |
|
|
(f) Hiesinger, K.; Dar'in, D.; Proschak, E.; Krasavin, M. J. Med. Chem. 2021, 64, 150.
doi: 10.1021/acs.jmedchem.0c01473 pmid: 33892142 |
|
|
(g) van Vlijmen, H.; Ortholand, J.-Y.; Li, V. M.; de Vlieger, J. S. B. Drug Discovery Today 2021, 26, 2406.
doi: 10.1016/j.drudis.2021.04.019 pmid: 33892142 |
|
|
(h) Konteatis, Z. Expert Opin. Drug Discovery 2021, 16, 723.
doi: 10.1080/17460441.2021.1905629 pmid: 33892142 |
|
| [5] |
(a) Wetzel, S.; Schuffenhauer, A.; Roggo, S.; Ertl, P.; Waldmann, H. CHIMIA 2007, 61, 355.
doi: 10.2533/chimia.2007.355 pmid: 32015480 |
|
(b) Lachance, H.; Wetzel, S.; Kumar, K.; Waldmann, H. J. Med. Chem. 2012, 55, 5989-6001.
doi: 10.1021/jm300288g pmid: 32015480 |
|
|
(c) Over, B.; Wetzel, S.; Grütter, C.; Nakai, Y.; Renner, S.; Rauh, D.; Waldmann, H. Nat. Chem. 2013, 5, 21.
doi: 10.1038/nchem.1506 pmid: 32015480 |
|
|
(d) Stratton, C. F.; Newman, D. J.; Tan, D. S. Bioorg. Med. Chem. Lett. 2015, 25, 4802.
doi: 10.1016/j.bmcl.2015.07.014 pmid: 32015480 |
|
|
(e) Ntie-Kang, F.; Nyongbela, K. D.; Ayimele, G. A.; Shekfeh, S. Phys. Sci. Rev. 2019, 4, 20180169.
pmid: 32015480 |
|
|
(f) Chen, Y.; Kirchmair, J. Mol. Inform. 2020, 39, e2000171.
pmid: 32015480 |
|
|
(g) Hanby, A. R.; Troelsen, N. S.; Osberger, T. J.; Kidd, S. L.; Mortensen, K. T.; Spring, D. R. Chem. Commun. 2020, 56, 2280.
doi: 10.1039/C9CC09796A pmid: 32015480 |
|
|
(h) Stone, S.; Newman, D. J.; Colletti, S. L.; Tan, D. S. Nat. Prod. Rep. 2021, 10.1039/d1np00039j.
doi: 10.1039/d1np00039j pmid: 32015480 |
|
|
(i) Karageorgis, G.; Reckzeh, E. S.; Ceballos, J.; Schwalfenberg, M.; Sievers, S.; Ostermann, C.; Pahl, A.; Ziegler, S.; Waldmann, H. Nat. Chem. 2018, 10, 1103.
doi: 10.1038/s41557-018-0132-6 pmid: 32015480 |
|
|
(j) Grigalunas, M.; Burhop, A.; Christoforow, A.; Waldmann, H. Curr. Opin. Chem. Biol. 2020, 56, 111.
doi: S1367-5931(19)30117-6 pmid: 32015480 |
|
|
(k) Karageorgis, G.; Foley, D. J.; Laraia, L.; Waldmann, H. Nat. Chem. 2020, 12, 227.
doi: 10.1038/s41557-019-0411-x pmid: 32015480 |
|
| [6] |
(a) Wei, W.; Rasul, A.; Sadiqa, A.; Sarfraz, I.; Hussain, G.; Nageen, B.; Liu, X.; Watanabe, N.; Selamoglu, Z.; Ali, M.; Li, X.; Li, J. Int. J. Biol. Sci. 2019, 15, 1600.
doi: 10.7150/ijbs.34716 pmid: 31360103 |
|
(b) Hashem, S.; Nisar, S.; Sageena, G.; Macha, M. A.; Yadav, S. K.; Krishnankutty, R.; Uddin, S.; Haris, M.; Bhat, A. A. Nutr. Cancer 2021, 73, 181.
doi: 10.1080/01635581.2020.1749676 pmid: 31360103 |
|
| [7] |
(a) Nadin, A.; Hattotuwagama, C.; Churcher, I. Angew. Chem. Int. Ed. 2012, 51, 1114.
doi: 10.1002/anie.201105840 pmid: 24239725 |
|
(b) Doveston, R.; Marsden, S.; Nelson, A. Drug Discovery Today 2014, 19, 813.
doi: 10.1016/j.drudis.2013.11.006 pmid: 24239725 |
|
| [8] |
Colomer, I.; Empson, C. J.; Craven, P.; Owen, Z.; Doveston, R. G.; Churcher, I.; Marsden, S. P.; Nelson, A. Chem. Commun. 2016, 52, 7209.
doi: 10.1039/C6CC03244C |
| [9] |
(a) Sun, H.-J.; Zou, Y.-P.; Nie, X.-F.; Yu, R.-H. Chin. J. Pharm. 1983, 14, 12. (in Chinese)
|
|
( 孙汉杰, 邹亚平, 聂秀范, 于润海, 中国医药工业杂志, 1983, 14, 12.)
|
|
|
(b) Zhao, J.-H. M.S. Thesis, Liaoning University, Shenyang, 2017. (in Chinese)
|
|
|
( 赵京华, 硕士论文, 辽宁大学, 沈阳, 2017.)
|
|
|
(c) Liu, J.; Zhao, J.; Zhang, J.; Cui, Q.; Wang, X.; Liang, X.; Liu, H.; Chen, L. J. Lat. Am. J. Pharm. 2017, 36, 955.
|
|
| [10] |
(a) Ma, X. M.S. Thesis, Dalian University of Technology, Dalian, 2010. (in Chinese)
|
|
( 马旭, 硕士论文, 大连理工大学,大连, 2010.)
|
|
|
(b) Guo, P.; Liu, J.-M.; Ye, F.-Q.; Li, X.-K.; Yao, Q.-Z. Chin. J. Org. Chem. 2012, 32, 2003. (in Chinese)
doi: 10.6023/cjoc201204007 |
|
|
( 郭平, 刘剑敏, 叶发青, 李校堃, 姚其正, 有机化学, 2012, 32, 2003.)
doi: 10.6023/cjoc201204007 |
|
| [11] |
(a) Guo, P.; Wang, Y.-W.; Weng, B.-X.; Li, X.-K.; Yang, S.-L.; Ye, F.-Q. J. Asian Nat. Prod. Res. 2014, 16, 53.
doi: 10.1080/10286020.2013.857660 |
|
(b) Guo, P. Ph.D. Dissertation, Nanjing University Of Science And Technology, Nanjing, 2015. (in Chinese)
|
|
|
( 郭平, 博士论文, 南京理工大学, 南京, 2015.)
|
|
|
(c) Meng, X.-W.; Nie, T.-Q.; Zhang, X.-X. CN 111153912, 2020.
|
|
| [12] |
Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315.
doi: 10.1021/acs.jmedchem.5b00258 pmid: 26200936 |
| [13] |
Jiang, H.; Studer, A. Angew. Chem., Int. Ed. 2018, 57, 10707.
doi: 10.1002/anie.201804966 |
| [14] |
CCDC 2156806 (12a) contains the supplementary crystallographic data for this paper. These data are free of charge from The Cambridge Crystallographic Centre via. www.ccdc.cam.ac.uk/data_request/cif.
|
| [15] |
(a) Lipinski, C. A.; Lombardo, F.; Dominy, B. W.; Feeney, P. J. Adv. Drug Delivery Rev. 2001, 46, 3.
doi: 10.1016/S0169-409X(00)00129-0 |
|
(b) Veber, D. F.; Johnson, S. R.; Cheng, H.-Y.; Smith, B. R.; Ward, K. W.; Kopple, K. D. J. Med. Chem. 2002, 45, 2615.
doi: 10.1021/jm020017n |
|
| [16] |
(a) Firth, N. C.; Brown, N.; Blagg, J. J. Chem. Inf. Model. 2012, 52, 2516.
doi: 10.1021/ci300293f |
|
(b) Bento, A. P.; Gaulton, A.; Hersey, A.; Bellis, L. J.; Chambers, J.; Davies, M.; Krüger, F. A.; Light, Y.; Mak, L.; McGlinchey, S.; Nowotka, M.; Papadatos, G.; Santos, R.; Overington, J. P. Nucleic Acids Res. 2014, 42, D1083.
doi: 10.1093/nar/gkt1031 |
|
|
(c) Meyers, J.; Carter, M.; Mok, N. Y.; Brown, N. Future Med. Chem. 2016, 8, 1753.
doi: 10.4155/fmc-2016-0095 |
| [1] | 刘改枝, 李金鑫, 史礼君, 刘萌芽, 蔡邦荣. 熊果酸的结构修饰与生物活性研究进展[J]. 有机化学, 2021, 41(8): 2974-2989. |
| [2] | 周汇源, 吴堰霖, 黄典红, 张铭婷, 陈欢明, 钱明成, 赵帅, 张辛燕, 陈新. 山竹醇新类似物的合成及其抗肿瘤活性研究[J]. 有机化学, 2020, 40(6): 1578-1587. |
| [3] | 董子阳, 杨占会, 许家喜. 马钱子碱的结构修饰和手性应用[J]. 有机化学, 2020, 40(12): 4101-4121. |
| [4] | 戴一, 仲飞. 冬凌草甲素的结构修饰与生物活性研究进展[J]. 有机化学, 2017, 37(7): 1701-1713. |
| [5] | 刘颖杰, 丁泽洋, 谭冲, 袁洪亮, 季宇彬. 石蒜碱的全合成及结构修饰研究进展[J]. 有机化学, 2015, 35(5): 1009-1021. |
| [6] | 唐初, 陈玉, 柏舜, 杨光忠. 齐墩果酸的结构修饰与生物活性研究进展[J]. 有机化学, 2013, 33(01): 46-65. |
| [7] | 郭平, 刘剑敏, 叶发青, 李校堃, 姚其正. 莪术醇在Woodward-Prévost反应条件下的产物研究[J]. 有机化学, 2012, 32(10): 2003-2006. |
| [8] | 王欢欢, 吴平, 康宏, 许亮, 朱瑞新, 康廷国. 嘧啶衍生物对牛蒡子苷元片段修饰的研究[J]. 有机化学, 2012, 32(10): 1894-1898. |
| [9] | 梁英, 喻大昭. 大黄素的化学合成及结构修饰研究进展[J]. 有机化学, 2011, 31(08): 1324-1333. |
| [10] | 樊会丹a ; 张从海a ; 严胜骄a ; 林 军*,a,b. 印楝素的合成、结构修饰及生物活性研究进展[J]. 有机化学, 2009, 29(01): 20-33. |
| [11] | 战佩英a ; 李东风b ; 王进军*,c. 环稠卟啉合成的研究进展[J]. 有机化学, 2008, 28(12): 2039-2056. |
| [12] | 赵圣印*; 邵志宇; 钦维民; 张灯青. 吲哚马来酰亚胺类蛋白激酶C抑制剂的研究进展[J]. 有机化学, 2008, 28(10): 1676-1684. |
| [13] | 王进军. 叶绿素-a衍生物的化学反应和多取代卟吩(啉)合成的研究进展[J]. 有机化学, 2005, 25(11): 1353-1371. |
| [14] | 周冰, 许新华, 张秋林, 陈茹玉. 磷脂核苷缀合物甘油结构修饰新方法及应用[J]. 有机化学, 2004, 24(1): 88-92. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||