有机化学 ›› 2022, Vol. 42 ›› Issue (7): 1960-1973.DOI: 10.6023/cjoc202202031 上一篇 下一篇
综述与进展
罗力铖a,b, 李亮a, 穆有炳b, 李博文b, 万晓波b,*( )
)
收稿日期:2022-02-25
修回日期:2022-04-07
发布日期:2022-08-09
通讯作者:
万晓波
基金资助:
Licheng Luoa,b, Liang Lia, Youbing Mub, Bowen Lib, Xiaobo Wanb( )
)
Received:2022-02-25
Revised:2022-04-07
Published:2022-08-09
Contact:
Xiaobo Wan
Supported by:文章分享
异靛蓝因其良好的平面性以及较强的拉电子能力, 一直作为优秀的受体材料在有机光电材料中扮演着重要的角色, 对异靛蓝的结构改造也成为了学术界关注的重点. 其中, 含氮异靛蓝衍生物的合成及其在光电材料中的应用取得了较大的进展, 氮原子的引入可进一步调节异靛蓝衍生物推拉电子的能力以及平面性, 从而调节其光电性能. 本综述总结了近年来含氮的芳杂环异靛蓝衍生物的发展过程以及在光电材料中的应用, 以期为功能化共轭分子的设计提供有益的借鉴和指导.
罗力铖, 李亮, 穆有炳, 李博文, 万晓波. 含氮异靛蓝衍生物的合成及其在光电材料中的应用[J]. 有机化学, 2022, 42(7): 1960-1973.
Licheng Luo, Liang Li, Youbing Mu, Bowen Li, Xiaobo Wan. Synthesis of Nitrogen-Containing Isoindigo Derivatives and Their Applications in Optoelectronic Materials[J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 1960-1973.
| Compd. | Voc/V | Jsc/(mA•cm–2) | FF/% | PCE/% |
|---|---|---|---|---|
| 24 | 0.66±0.05 | 2.7±0.3 | 31±3 | 0.74 |
| 25 | 0.28±0.09 | 0.22±0.04 | 28±2 | 0.03 |
| 26 | 0.67±0.01 | 2.5±0.9 | 40±3 | 0.93 |
| 27 | 0.3±0.1 | 0.6±0.2 | 22±2 | 0.07 |
| Compd. | Voc/V | Jsc/(mA•cm–2) | FF/% | PCE/% |
|---|---|---|---|---|
| 24 | 0.66±0.05 | 2.7±0.3 | 31±3 | 0.74 |
| 25 | 0.28±0.09 | 0.22±0.04 | 28±2 | 0.03 |
| 26 | 0.67±0.01 | 2.5±0.9 | 40±3 | 0.93 |
| 27 | 0.3±0.1 | 0.6±0.2 | 22±2 | 0.07 |
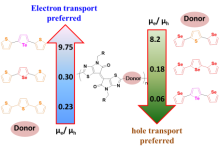
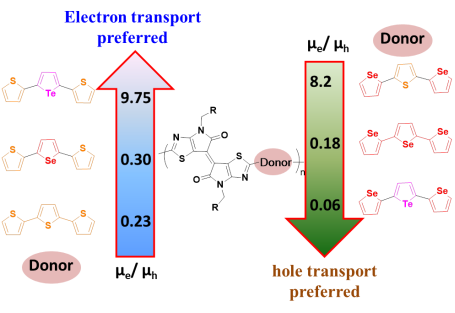
| Polymer | Mn (kDa)/PDI | HOMO/eV | LUMO/eV | μh/(cm2•V–1•s–1) | μe/(cm2•V–1•s–1) | Ion/Ioff | Device structure |
|---|---|---|---|---|---|---|---|
| IIDDT-C3 | 20.4/2.0 | –5.70 | –3.70 | 3.62 (2.98) | N/A | 106 | BG/TC |
| PAIID-BT-C1 | 61.1/3.0 | –5.66 | –3.62 | 3.63 (2.86) | N/A | N/A | BG/BC |
| PAIID-BT-C3 | 50.6/3.87 | –5.67 | –3.64 | 7.28 (6.43) | N/A | N/A | BG/BC |
| PAIID-BT-C1 | 61.1/3.0 | –5.66 | –3.62 | 0.45 (0.27) | 0.47 (0.30) | N/A | TG/BC |
| PAIID-BT-C3 | 50.6/3.87 | –5.67 | –3.64 | 2.33 (1.89) | 0.78 (0.48) | N/A | TG/BC |
| 5DNIID-2T | 33.46/2.68 | –5.92 | –3.82 | 1.27×10–3 | N/A | N/A | BG/TC |
| PAIIDBT | 14/1.6 | –5.5 | –3.4 | N/A | 1.0 | 106 | TG/BC |
| PAIIDSe | 96/2.0 | –5.3 | –3.3 | 0.2 | 0.5 | 102~103 | TG/BC |
| PAIID-TT-C3 | 19.8/2.63 | –5.68 | –3.70 | 1.14 (0.78) | 1.54 (1.12) | 10~102 | TG/BC |
| PAIID-DTE-C3 | 116.4/2.49 | –5.56 | –3.60 | 1.91 (1.59) | 0.04 (0.02) | 103~104 | TG/BC |
| PAIID-BS-C3 | 37.7/3.92 | –5.58 | –3.66 | 2.56 (2.38) | 0.44 (0.32) | 103~104 | TG/BC |
| PAIID-DSE-C3 | 42.4/4.24 | –5.54 | –3.65 | 0.80 (0.73) | 0.18 (0.14) | 103~104 | TG/BC |
| BDPPV | 41.8/2.39 | –6.20 | –4.01 | N/A | 0.5 | 104 | TG/BC |
| BDOPV-2T | 77.2/3.00 | –5.72 | –4.15 | N/A | 1.74(1.42) | N/A | TG/BC |
| AzaBDOPV-2T | 135/2.62 | –5.77 | –4.45 | N/A | 3.22 (1.63) | N/A | TG/BC |
| PBABDF-DT | 32.6/5.0 | –5.70 | –4.04 | 1.86 (1.34) | N/A | 106 | BG/TC |
| PBABDF-TVT | 30.8/5.83 | –5.60 | –3.99 | 1.56 (1.17) | N/A | 1.0×106 | BG/TC |
| PNBDOPV-DTBT | 29.9/2.68 | –5.87 | –3.92 | 5.97(4.70) | 7.07 (4.75) | N/A | TG/BC |
| PNBDOPV-DTF2BT | 31.31/2.71 | –6.05 | –3.92 | 0.83 (0.80) | 3.77 (3.14) | N/A | TG/BC |
| PAIID-TFBVB-C1 | 26.06/1.48 | –6.23 | –3.69 | N/A | 5.9×10–4 (3.6×10–4) | 104 | TG/BC |
| PAIID-TFBVB-C3 | 32.16/1.95 | –6.18 | –3.61 | N/A | 8.8×10–4 (5.9×10–4) | 105 | TG/BC |
| PAIID-BVB-C3 | 55.3/2.32 | –5.73 | –3.47 | 0.047 (0.036) | N/A | 105 | TG/BC |
| PAZDTBT | 19.3/1.94 | –5.63 | –3.53 | 0.377 (0.496) | 0.092 (0.137) | 103~104 | TG/BC |
| PBABDF-TVT | 30.8/5.83 | –5.60 | –3.99 | 1.56 (1.17) | N/A | 1.0×106 | BG/TC |
| PNBDOPV-DTBT | 29.9/2.68 | –5.87 | –3.92 | 5.97 (4.70) | 7.07 (4.75) | N/A | TG/BC |
| PAZDTBTF | 13.5/1.71 | –5.78 | –3.56 | 0.358 (0.417) | 0.126 (0.143) | 103~104 | TG/BC |
| PBAID1-BT | 39.5/2.5 | –5.15 | –3.87 | 1.8×10–2 | N/A | N/A | BG/TC |
| Polymer | Mn(kDa)/PDI | HOMO/eV | LUMO/eV | μh/(cm2•V–1•s–1) | μe/(cm2•V–1•s–1) | Ion/Ioff | Device structure |
| PBAID2-BT | 46.8/2.3 | –5.16 | –3.87 | 1.33 | 0.94 | 102~103 | BG/TC |
| PBAID3-BT | 42.3/2.7 | –5.15 | –3.86 | 1.66 | 0.88 | 102~103 | BG/TC |
| PBAID3-2FBT | 40.6/2.6 | –5.17 | –3.90 | 1.68 | 1.37 | 103~104 | BG/TC |
| PNBDO-TT | 54.3/2.36 | –5.99 | –4.07 | N/A | 2.08 | 103~104 | N/A |
| PNBDO-DMTT | 43.7/1.72 | –5.94 | –4.00 | 0.0255 | 0.739 | 103~104 | N/A |
| PNBDO-MOTT | 34.5/1.93 | –5.75 | –4.00 | 0.0711 | 0.103 | 103~104 | N/A |
| P2N2F-BT | 35.3/2.88 | –5.67 | –3.88 | 0.30 (0.19) | 1.51 (1.28) | 103 | TG/BC |
| P2N2F-4FBT | 20.3/2.20 | –6.04 | –4.01 | N/A | 1.24 (1.08) | 105 | TG/BC |
| P(TzII-TTT) | 71.1/N/A | –5.13 | –3.58 | 3.93 (3.31) | 1.07 (0.76) | 102 | TG/BC |
| P(TzII-TTT) | 71.1/N/A | –5.13 | –3.58 | 4.34 (3.20) | N/A | 102 | BG/TC |
| P(TzII-T-Se-T) | 103.7/N/A | –5.12 | –3.53 | 3.77 (2.73) | 1.59 (0.83) | 102 | TG/BC |
| P(TzII-T-Te-T) | 19.4/N/A | –5.11 | –3.55 | 0.04 (0.04) | 0.64 (0.39) | 10 | TG/BC |
| P(TzII-Se-T-Se) | 28.8/N/A | –5.17 | –3.60 | 0.12 (0.05) | 0.58 (0.41) | 10 | TG/BC |
| P(TzII-Se-Se-Se) | 28.1/N/A | –5.20 | –3.59 | 1.26 (0.72) | 0.15 (0.13) | 102 | TG/BC |
| P(TzII-Se-Te-Se) | 8.3/N/A | –5.21 | –3.61 | 0.90 (0.66) | 0.08 (0.04) | 102 | TG/BC |
| Polymer | Mn (kDa)/PDI | HOMO/eV | LUMO/eV | μh/(cm2•V–1•s–1) | μe/(cm2•V–1•s–1) | Ion/Ioff | Device structure |
|---|---|---|---|---|---|---|---|
| IIDDT-C3 | 20.4/2.0 | –5.70 | –3.70 | 3.62 (2.98) | N/A | 106 | BG/TC |
| PAIID-BT-C1 | 61.1/3.0 | –5.66 | –3.62 | 3.63 (2.86) | N/A | N/A | BG/BC |
| PAIID-BT-C3 | 50.6/3.87 | –5.67 | –3.64 | 7.28 (6.43) | N/A | N/A | BG/BC |
| PAIID-BT-C1 | 61.1/3.0 | –5.66 | –3.62 | 0.45 (0.27) | 0.47 (0.30) | N/A | TG/BC |
| PAIID-BT-C3 | 50.6/3.87 | –5.67 | –3.64 | 2.33 (1.89) | 0.78 (0.48) | N/A | TG/BC |
| 5DNIID-2T | 33.46/2.68 | –5.92 | –3.82 | 1.27×10–3 | N/A | N/A | BG/TC |
| PAIIDBT | 14/1.6 | –5.5 | –3.4 | N/A | 1.0 | 106 | TG/BC |
| PAIIDSe | 96/2.0 | –5.3 | –3.3 | 0.2 | 0.5 | 102~103 | TG/BC |
| PAIID-TT-C3 | 19.8/2.63 | –5.68 | –3.70 | 1.14 (0.78) | 1.54 (1.12) | 10~102 | TG/BC |
| PAIID-DTE-C3 | 116.4/2.49 | –5.56 | –3.60 | 1.91 (1.59) | 0.04 (0.02) | 103~104 | TG/BC |
| PAIID-BS-C3 | 37.7/3.92 | –5.58 | –3.66 | 2.56 (2.38) | 0.44 (0.32) | 103~104 | TG/BC |
| PAIID-DSE-C3 | 42.4/4.24 | –5.54 | –3.65 | 0.80 (0.73) | 0.18 (0.14) | 103~104 | TG/BC |
| BDPPV | 41.8/2.39 | –6.20 | –4.01 | N/A | 0.5 | 104 | TG/BC |
| BDOPV-2T | 77.2/3.00 | –5.72 | –4.15 | N/A | 1.74(1.42) | N/A | TG/BC |
| AzaBDOPV-2T | 135/2.62 | –5.77 | –4.45 | N/A | 3.22 (1.63) | N/A | TG/BC |
| PBABDF-DT | 32.6/5.0 | –5.70 | –4.04 | 1.86 (1.34) | N/A | 106 | BG/TC |
| PBABDF-TVT | 30.8/5.83 | –5.60 | –3.99 | 1.56 (1.17) | N/A | 1.0×106 | BG/TC |
| PNBDOPV-DTBT | 29.9/2.68 | –5.87 | –3.92 | 5.97(4.70) | 7.07 (4.75) | N/A | TG/BC |
| PNBDOPV-DTF2BT | 31.31/2.71 | –6.05 | –3.92 | 0.83 (0.80) | 3.77 (3.14) | N/A | TG/BC |
| PAIID-TFBVB-C1 | 26.06/1.48 | –6.23 | –3.69 | N/A | 5.9×10–4 (3.6×10–4) | 104 | TG/BC |
| PAIID-TFBVB-C3 | 32.16/1.95 | –6.18 | –3.61 | N/A | 8.8×10–4 (5.9×10–4) | 105 | TG/BC |
| PAIID-BVB-C3 | 55.3/2.32 | –5.73 | –3.47 | 0.047 (0.036) | N/A | 105 | TG/BC |
| PAZDTBT | 19.3/1.94 | –5.63 | –3.53 | 0.377 (0.496) | 0.092 (0.137) | 103~104 | TG/BC |
| PBABDF-TVT | 30.8/5.83 | –5.60 | –3.99 | 1.56 (1.17) | N/A | 1.0×106 | BG/TC |
| PNBDOPV-DTBT | 29.9/2.68 | –5.87 | –3.92 | 5.97 (4.70) | 7.07 (4.75) | N/A | TG/BC |
| PAZDTBTF | 13.5/1.71 | –5.78 | –3.56 | 0.358 (0.417) | 0.126 (0.143) | 103~104 | TG/BC |
| PBAID1-BT | 39.5/2.5 | –5.15 | –3.87 | 1.8×10–2 | N/A | N/A | BG/TC |
| Polymer | Mn(kDa)/PDI | HOMO/eV | LUMO/eV | μh/(cm2•V–1•s–1) | μe/(cm2•V–1•s–1) | Ion/Ioff | Device structure |
| PBAID2-BT | 46.8/2.3 | –5.16 | –3.87 | 1.33 | 0.94 | 102~103 | BG/TC |
| PBAID3-BT | 42.3/2.7 | –5.15 | –3.86 | 1.66 | 0.88 | 102~103 | BG/TC |
| PBAID3-2FBT | 40.6/2.6 | –5.17 | –3.90 | 1.68 | 1.37 | 103~104 | BG/TC |
| PNBDO-TT | 54.3/2.36 | –5.99 | –4.07 | N/A | 2.08 | 103~104 | N/A |
| PNBDO-DMTT | 43.7/1.72 | –5.94 | –4.00 | 0.0255 | 0.739 | 103~104 | N/A |
| PNBDO-MOTT | 34.5/1.93 | –5.75 | –4.00 | 0.0711 | 0.103 | 103~104 | N/A |
| P2N2F-BT | 35.3/2.88 | –5.67 | –3.88 | 0.30 (0.19) | 1.51 (1.28) | 103 | TG/BC |
| P2N2F-4FBT | 20.3/2.20 | –6.04 | –4.01 | N/A | 1.24 (1.08) | 105 | TG/BC |
| P(TzII-TTT) | 71.1/N/A | –5.13 | –3.58 | 3.93 (3.31) | 1.07 (0.76) | 102 | TG/BC |
| P(TzII-TTT) | 71.1/N/A | –5.13 | –3.58 | 4.34 (3.20) | N/A | 102 | BG/TC |
| P(TzII-T-Se-T) | 103.7/N/A | –5.12 | –3.53 | 3.77 (2.73) | 1.59 (0.83) | 102 | TG/BC |
| P(TzII-T-Te-T) | 19.4/N/A | –5.11 | –3.55 | 0.04 (0.04) | 0.64 (0.39) | 10 | TG/BC |
| P(TzII-Se-T-Se) | 28.8/N/A | –5.17 | –3.60 | 0.12 (0.05) | 0.58 (0.41) | 10 | TG/BC |
| P(TzII-Se-Se-Se) | 28.1/N/A | –5.20 | –3.59 | 1.26 (0.72) | 0.15 (0.13) | 102 | TG/BC |
| P(TzII-Se-Te-Se) | 8.3/N/A | –5.21 | –3.61 | 0.90 (0.66) | 0.08 (0.04) | 102 | TG/BC |
| [1] |
Mei, J.; Graham, K. R.; Stalder, R.; Reynolds, J. R. Org. Lett. 2010, 12, 660.
doi: 10.1021/ol902512x |
| [2] |
Sariciftci, N. S.; Smilowitz, L.; Heeger, A. J.; Wudl, F. Sciene 1992, 258, 1474.
doi: 10.1126/science.258.5087.1474 |
| [3] |
Wang, E.; Ma, Z.; Zhang, Z.; Henriksson, P.; Inganäs, O.; Zhang, F.; Andersson, M. R. Chem. Commun. 2011, 47, 4908.
doi: 10.1039/c1cc11053e |
| [4] |
Lei, T.; Dou, J. H.; Pei, J. Adv. Mater. 2012, 24, 6457.
doi: 10.1002/adma.201202689 |
| [5] |
Wang, E.; Mammo, W.; Andersson, M. R. Adv. Mater. 2014, 26, 1801.
doi: 10.1002/adma.201304945 |
| [6] |
Lei, T.; Cao, Y.; Fan, Y.; Liu, C. J.; Yuan, S. C.; Pei, J. J. Am. Chem. Soc. 2011, 133, 6099.
doi: 10.1021/ja111066r |
| [7] |
Heeger, A. J. Chem. Soc. Rev. 2010, 39, 2354.
doi: 10.1039/b914956m pmid: 20571667 |
| [8] |
Yuen, J. D.; Wudl, F. Energy Environ. Sci. 2013, 6, 392.
doi: 10.1039/c2ee23505f |
| [9] |
Tsumura, A.; Koezuka, H.; Ando, T. J. A. P. L. Appl. Phys. Lett. 1986, 49, 1210.
doi: 10.1063/1.97417 |
| [10] |
Tang, C. W.; VanSlyke, S. A. Appl. Phys. Lett. 1987, 51, 913.
doi: 10.1063/1.98799 |
| [11] |
Tang, C. W. Appl. Phys. Lett. 1986, 48, 183.
doi: 10.1063/1.96937 |
| [12] |
Wei, X.; Zhang, W.; Yu, G. Adv. Funct. Mater. 2021, 31, 2010979.
doi: 10.1002/adfm.202010979 |
| [13] |
Lu, Y.; Ding, Y.; Wang, J.; Pei, J. Chin. J. Org. Chem. 2016, 36, 2272. (in Chinese)
doi: 10.6023/cjoc201606015 |
|
( 卢阳, 丁一凡, 王婕妤, 裴坚, 有机化学, 2016, 36, 2272.)
doi: 10.6023/cjoc201606015 |
|
| [14] |
Ashraf, R. S.; Kronemeijer, A. J.; James, D. I.; Sirringhaus, H.; McCulloch, I. Chem. Commun. 2012, 48, 3939.
doi: 10.1039/c2cc30169e |
| [15] |
Kritsanida, M.; Magiatis, P.; Skaltsounis, A. L.; Peng, Y.; Li, P.; Wennogle, L. P. J. Nat. Prod. 2009, 72, 2199.
doi: 10.1021/np9003905 pmid: 19994845 |
| [16] |
de Miguel, G.; Camacho, L.; García-Frutos, E. M. J. Mater. Chem. C. 2016, 4, 1208.
doi: 10.1039/C5TC03464G |
| [17] |
Marfat, A.; Carta, M. P. Tetrahedron Lett. 1987, 28, 4027.
|
| [18] |
Sriram, R.; Sesha Sai Pavan Kumar, C. N.; Raghunandan, N.; Ramesh, V.; Sarangapani, M.; Rao, V. J. Synth. Commun. 2012, 42, 3419.
doi: 10.1080/00397911.2011.584008 |
| [19] |
Huang, J.; Mao, Z.; Chen, Z.; Gao, D.; Wei, C.; Zhang, W.; Yu, G. Chem. Mater. 2016, 28, 2209.
doi: 10.1021/acs.chemmater.6b00154 |
| [20] |
Lu, Y.; Liu, Y.; Dai, Y. Z.; Yang, C. Y.; Un, H. I.; Liu, S. W.; Pei, J. Chem.-Asian J. 2017, 12, 302.
doi: 10.1002/asia.201601671 |
| [21] |
Randell, N. M.; Douglas, A. F.; Kelly, T. L. J. Mater. Chem. A 2014, 2, 1085.
doi: 10.1039/C3TA14263A |
| [22] |
Randell, N. M.; Fransishyn, K. M.; Kelly, T. L. ACS Appl. Mater. Interfaces 2017, 9, 24788.
doi: 10.1021/acsami.7b06335 |
| [23] |
Ashizawa, M.; Hasegawa, T.; Kawauchi, S.; Masunaga, H.; Hikima, T.; Sato, H.; Matsumoto, H. RSC Adv. 2016, 6, 109434.
doi: 10.1039/C6RA17424H |
| [24] |
Garzón, A.; Navarro, A.; López, D.; Perles, J.; García-Frutos, E. M. J. Phys. Chem. C 2017, 121, 27071.
doi: 10.1021/acs.jpcc.7b07625 |
| [25] |
Yue, W.; Nikolka, M.; Xiao, M.; Sadhanala, A.; Nielsen, C. B.; White, A. J.; McCulloch, I. J. Mater. Chem. 2016, 4, 9704.
|
| [26] |
Parr, Z. S.; Borges-González, J.; Rashid, R. B.; Thorley, K. J.; Meli, D.; Paulsen, B. D.; Nielsen, C. B. Adv. Mater. 2107829.
|
| [27] |
Paulsen, B. D.; Tybrandt, K.; Stavrinidou, E.; Rivnay, J. Nat. Mater. 2020, 19, 13
doi: 10.1038/s41563-019-0435-z pmid: 31427743 |
| [28] |
Yue, W.; Li, C.; Tian, X.; Li, W.; Neophytou, M.; Chen, H.; McCulloch, I. J. Polym. Sci., art A: Polym. Chem. 2017, 55, 2691.
|
| [29] |
Huang, J.; Chen, Z.; Mao, Z.; Gao, D.; Wei, C.; Lin, Z.; Yu, G. Adv. Electron. Mater. 2017, 3, 1700078.
doi: 10.1002/aelm.201700078 |
| [30] |
Zhou, Y.; Wang, Q.; Zhang, W.; Li, Y.; Huang, J.; Wei, C.; Yu, G. Dyes Pigm. 2020, 180, 108438.
doi: 10.1016/j.dyepig.2020.108438 |
| [31] |
Wei, C.; Zhang, W.; Huang, J.; Li, H.; Zhou, Y.; Yu, G. Macromolecules 2019, 52, 2911.
doi: 10.1021/acs.macromol.9b00022 |
| [32] |
Lei, T.; Dou, J. H.; Cao, X. Y.; Wang, J. Y.; Pei, J. J. Am. Chem. Soc. 2013, 135, 12168.
doi: 10.1021/ja403624a |
| [33] |
Shi, K.; Zhang, F.; Di, C. A.; Yan, T. W.; Zou, Y.; Zhou, X.; Pei, J. J. Am. Chem. Soc. 2015, 137, 6979.
doi: 10.1021/jacs.5b00945 pmid: 25997085 |
| [34] |
Lei, T.; Dou, J. H.; Cao, X. Y.; Wang, J. Y.; Pei, J. Adv. Mater. 2013, 25, 6589.
doi: 10.1002/adma.201302278 |
| [35] |
Dai, Y. Z.; Ai, N.; Lu, Y.; Zheng, Y. Q.; Dou, J. H.; Shi, K.; Pei, J. Chem. Sci. 2016, 7, 5753.
doi: 10.1039/C6SC01380E |
| [36] |
Zhang, G.; Dai, Y.; Song, K.; Lee, H.; Ge, F.; Qiu, L.; Cho, K. Polym. Chem. 2017, 8, 2381.
doi: 10.1039/C7PY00295E |
| [37] |
Shi, K.; Zhang, W.; Gao, D.; Zhang, S.; Lin, Z.; Zou, Y.; Yu, G. Adv. Mater. 2018, 30, 1705286.
doi: 10.1002/adma.201705286 |
| [38] |
Chen, Z.; Huang, J.; Zhang, W.; Zhou, Y.; Wei, X.; Wei, J.; Yu, G. J. Mater. Chem. C 2022, 10, 2671.
doi: 10.1039/D1TC02833B |
| [39] |
Chen, F.; Jiang, Y.; Sui, Y.; Zhang, J.; Tian, H.; Han, Y.; Geng, Y. Macromolecules 2018, 51, 8652.
doi: 10.1021/acs.macromol.8b01885 |
| [40] |
Liu, H.; Zhang, X.; Cheng, J.; Ye, D.; Chen, L.; Wen, H.; Liu, S. Chin. J. Org. Chem. 2020, 40, 831. (in Chinese)
doi: 10.6023/cjoc201910042 |
|
( 刘慧, 张小凤, 程敬招, 叶东鼐, 陈龙, 温和瑞, 刘诗咏, 有机化学, 2020, 40, 831.)
doi: 10.6023/cjoc201910042 |
|
| [41] |
Huang, K.; Zhao, X.; Du, Y.; Kim, S.; Wang, X.; Lu, H.; Qiu, L. J. Mater. Chem. C 2019, 7, 7618.
doi: 10.1039/C9TC02021G |
| [42] |
Randell, N. M.; Radford, C. L.; Yang, J.; Quinn, J.; Hou, D.; Li, Y.; Kelly, T. L. Chem. Mater. 2018, 30, 4864
doi: 10.1021/acs.chemmater.8b02535 |
| [43] |
Nishi, H.; Kitahara, K.; Tokita, S. Nippon Kagaku Kaishi 1977, 146.
|
| [44] |
Wang, X.; Zhao, Z.; Ai, N.; Pei, J.; Liu, Y.; Wan, X. Eur. J. Org. Chem. 2016, 15, 2603.
|
| [45] |
Lin, Y.; Fan, H.; Li, Y.; Zhan, X. Adv. Mater. 2012, 24, 3087.
doi: 10.1002/adma.201200721 |
| [46] |
Li, C.; Zhang, H.; Mirie, S.; Peng, J.; Cai, M.; Wang, X.; Wan, X. Org. Chem. Front. 2018, 5, 442.
doi: 10.1039/C7QO00841D |
| [47] |
Ashraf, R. S.; Kronemeijer, A. J.; James, D. I.; Sirringhaus, H.; McCulloch, I. Chem. Commun. 2012, 48, 3939.
doi: 10.1039/c2cc30169e |
| [48] |
Cao, Y.; Yuan, J.-S.; Zhou, X.; Wang, X.-Y.; Zhuang, F.-D.; Wang, J.-Y.; Pei, J. Chem. Commun. 2015, 51, 10514.
doi: 10.1039/C5CC02026C |
| [49] |
Li, C.; Un, H. I.; Peng, J.; Cai, M.; Wang, X.; Wang, J.; Wan, X. Chem.-Eur. J. 2018, 24, 9698.
doi: 10.1002/chem.201802608 |
| [50] |
Li, C. C.; Xiong, M.; Peng, J. W.; Wang, J. Y.; Zhang, H. R.; Mu, Y. B.; Wan, X. B. Chin. J. Polym. Sci. 2021, 39, 838.
doi: 10.1007/s10118-021-2552-9 |
| [1] | 梁龙, 刘丽娜, 陈学强, 项宣, 凌君, 鲁郑全, 李靖靖, 李维实. 苯并二噻吩/苯并噻二唑ADA型光电化合物:氟取代的影响[J]. 有机化学, 2019, 39(1): 157-169. |
| [2] | 石燕君, 倪振杰, 甄永刚, 董焕丽, 胡文平. 碳-氢键活化在有机半导体合成方面的应用[J]. 有机化学, 2016, 36(8): 1741-1764. |
| [3] | 侯方展, 梅崇余, 梁龙, 王洪宇, 谢光辉, 鲁郑全, 李靖靖, 李维实. 苯并二噻吩为核的小分子光电化合物:共轭链段拓展方向的影响[J]. 有机化学, 2016, 36(7): 1586-1595. |
| [4] | 卢阳, 丁一凡, 王婕妤, 裴坚. 基于异靛青的聚合物场效应晶体管材料研究进展[J]. 有机化学, 2016, 36(10): 2272-2283. |
| [5] | 李金玲, 谢宝粘, 彭进. 新型多环芳烃Bisanthene衍生物的合成研究进展[J]. 有机化学, 2015, 35(7): 1441-1450. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||