有机化学 ›› 2023, Vol. 43 ›› Issue (2): 668-678.DOI: 10.6023/cjoc202208029 上一篇 下一篇
研究论文
王雷刚a, 郑逸轩a, 周希a, 王海峰a, 严琼姣a, 汪伟a,*( ), 陈芬儿a,b,*(
), 陈芬儿a,b,*( )
)
收稿日期:2022-08-23
修回日期:2022-10-03
发布日期:2022-10-31
基金资助:
Leigang Wanga, Yixuan Zhenga, Xi Zhoua, Haifeng Wanga, Qiongjiao Yana, Wei Wanga( ), Fener Chena,b(
), Fener Chena,b( )
)
Received:2022-08-23
Revised:2022-10-03
Published:2022-10-31
Contact:
*E-mail: Supported by:文章分享
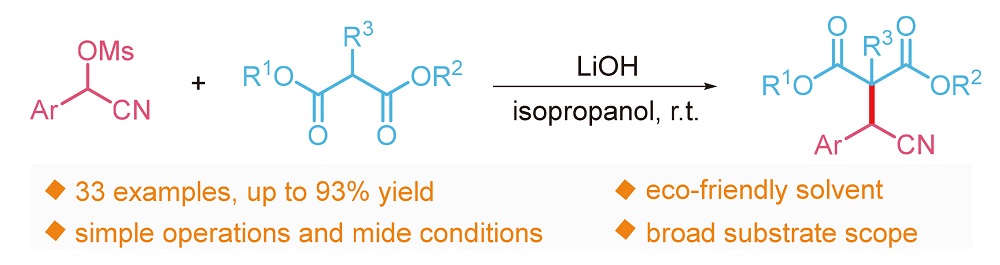
报道了无过渡金属存在下α-氰醇甲磺酸酯与丙二酸酯的亲核取代反应. 该方法的原料廉价易得, 操作简便, 反应条件温和, 具有良好的底物适用性和官能团兼容性, 以较好的产率得到了一系列重要的丙二酸酯取代的α-芳基腈类化合物. 更重要的是, 该方法也适用于非对称的丙二酸酯和酰基酯类底物.
王雷刚, 郑逸轩, 周希, 王海峰, 严琼姣, 汪伟, 陈芬儿. α-氰醇甲磺酸酯与丙二酸酯的亲核取代反应合成α-芳基腈类化合物[J]. 有机化学, 2023, 43(2): 668-678.
Leigang Wang, Yixuan Zheng, Xi Zhou, Haifeng Wang, Qiongjiao Yan, Wei Wang, Fener Chen. Synthesis of α-Aryl Nitriles via Nucleophilic Substitution of α-Cyanohydrin Methanesulfonates with Malonates[J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 668-678.
| Entry | LG | Base | Solvent | Yieldb/% |
|---|---|---|---|---|
| 1 | OMs | LiOt-Bu | THF | 50 |
| 2 | OAc | LiOt-Bu | THF | 0 |
| 3 | OBoc | LiOt-Bu | THF | 0 |
| 4 | OPiv | LiOt-Bu | THF | 0 |
| 5 | OTs | LiOt-Bu | THF | 40 |
| 6 | OMs | LiOH | THF | 52 |
| 7 | OMs | NaOH | THF | 15 |
| 8 | OMs | DBU | THF | 0 |
| 9 | OMs | NaHMDS | THF | 20 |
| 10 | OMs | LiOH | 1,4-Dioxane | 72 |
| 11 | OMs | LiOH | MeCN | 76 |
| 12 | OMs | LiOH | EtOAc | 53 |
| 13 | OMs | LiOH | Toluene | 24 |
| 14 | OMs | LiOH | IPA | 88 |
| 15 | OMs | - | IPA | 0 |
| 16 c | OMs | LiOH | IPA | 59 |
| 17 d | OMs | LiOH | IPA | 95 |
| Entry | LG | Base | Solvent | Yieldb/% |
|---|---|---|---|---|
| 1 | OMs | LiOt-Bu | THF | 50 |
| 2 | OAc | LiOt-Bu | THF | 0 |
| 3 | OBoc | LiOt-Bu | THF | 0 |
| 4 | OPiv | LiOt-Bu | THF | 0 |
| 5 | OTs | LiOt-Bu | THF | 40 |
| 6 | OMs | LiOH | THF | 52 |
| 7 | OMs | NaOH | THF | 15 |
| 8 | OMs | DBU | THF | 0 |
| 9 | OMs | NaHMDS | THF | 20 |
| 10 | OMs | LiOH | 1,4-Dioxane | 72 |
| 11 | OMs | LiOH | MeCN | 76 |
| 12 | OMs | LiOH | EtOAc | 53 |
| 13 | OMs | LiOH | Toluene | 24 |
| 14 | OMs | LiOH | IPA | 88 |
| 15 | OMs | - | IPA | 0 |
| 16 c | OMs | LiOH | IPA | 59 |
| 17 d | OMs | LiOH | IPA | 95 |
| [1] |
(a) Fleming, F. F. Nat. Prod. Rep. 1999, 16, 597.
doi: 10.1039/a804370a pmid: 20804202 |
|
(b) Fleming, F. F.; Yao, L.; Ravikumar, P. C.; Funk, L.; Shook, B. C. J. Med. Chem. 2010, 53, 7902.
doi: 10.1021/jm100762r pmid: 20804202 |
|
|
(c) Wang, J.; Liu, H. Chin. J. Org. Chem. 2012, 32, 1643. (in Chinese)
doi: 10.6023/cjoc1202132 pmid: 20804202 |
|
|
(王江, 柳红, 有机化学, 2012, 32, 1643.)
doi: 10.6023/cjoc1202132 pmid: 20804202 |
|
|
(d) Cohen, D. T.; Buchwald, S. L. Org. Lett. 2015, 17, 202.
doi: 10.1021/ol5032359 pmid: 20804202 |
|
|
(e) Yan, G.; Zhang, Y.; Wang, J. Adv. Synth. Catal. 2017, 359, 4068.
doi: 10.1002/adsc.201700875 pmid: 20804202 |
|
|
(f) Que, X.; Qiu, Z.; Yan, Y. High Perform. Polym. 2019, 31, 1062.
doi: 10.1177/0954008318821710 pmid: 20804202 |
|
| [2] |
(a) Daw, P.; Sinha, A.; Rahaman, S. M. W.; Dinda, S.; Bera, J. K. Organometallics 2012, 31, 3790.
doi: 10.1021/om300297y |
|
(b) Kumar, S.; Dixit, S. K.; Awasthi, S. K. Tetrahedron Lett. 2014, 55, 3802.
doi: 10.1016/j.tetlet.2014.05.050 |
|
|
(c) Tomás-Mendivil, E.; Suárez, F. J.; Díez, J.; Cadierno, V. Chem. Commun. 2014, 50, 9661.
doi: 10.1039/C4CC04058A |
|
|
(d) Manikandan, R.; Anitha, P.; Prakash, G.; Vijayan, P.; Viswanathamurthi, P.; Butcher, R. J.; Malecki, J. G. J. Mol. Catal. A: Chem. 2015, 398, 312.
doi: 10.1016/j.molcata.2014.12.017 |
|
|
(e) Marcé, P.; Lynch, J.; Blacker, A. J.; Williams, J. M. J. Chem. Commun. 2016, 52, 1436.
doi: 10.1039/C5CC08714G |
|
|
(f) Singh, K.; Sarbajna, A.; Dutta, I.; Pandey, P.; Bera, J. K. Chem. Eur. J. 2017, 23, 7761.
doi: 10.1002/chem.201700816 |
|
|
(g) Ji, P.; Manna, K.; Lin, Z.; Feng, X.; Urban, A.; Song, Y.; Lin, W. J. Am. Chem. Soc. 2017, 139, 7004.
doi: 10.1021/jacs.7b02394 |
|
|
(h) Paul, A.; Chandak, H. S.; Ma, L.; Seidel, D. Org. Lett. 2020, 22, 976.
doi: 10.1021/acs.orglett.9b04506 |
|
|
(i) Li, C.; Chang, X.-Y.; Huo, L.; Tan, H.; Xing, X.; Xu, C. ACS Catal. 2021, 11, 8716.
doi: 10.1021/acscatal.1c02254 |
|
|
(j) Tong, S.; Li, K.; Ouyang, X.; Song, R.; Li, J. Green Synth. Catal. 2021, 2, 145.
|
|
| [3] |
Jackson, T.; Woo, L. W. L.; Trusselle, M. N.; Chander, S. K.; Purohit, A.; Reed, M. J.; Potter, B. V. L. Org. Biomol. Chem. 2007, 5, 2940.
pmid: 17728860 |
| [4] |
Teodori, E.; Dei, S.; Garnier-Suillerot, A.; Gualtieri, F.; Manetti, D.; Martelli, C.; Romanelli, M. N.; Scapecchi, S.; Sudwan, P.; Salerno, M. J. Med. Chem. 2005, 48, 7426.
doi: 10.1021/jm050542x |
| [5] |
Noble, S.; McTavish, D. Drugs 1995, 50, 1032.
pmid: 8612470 |
| [6] |
Culkin, D. A.; Hartwig, J. F. J. Am. Chem. Soc. 2002, 124, 9330.
pmid: 12167001 |
| [7] |
Jiao, Z.; Chee, K. W.; Zhou, J. S. J. Am. Chem. Soc. 2016, 138, 16240.
doi: 10.1021/jacs.6b11610 |
| [8] |
Qian, X.; Han, J.; Wang, L. Adv. Synth. Catal. 2016, 358, 940.
doi: 10.1002/adsc.201501013 |
| [9] |
He, A.; Falck, J. R. J. Am. Chem. Soc. 2010, 132, 2524.
doi: 10.1021/ja910582n |
| [10] |
Nambo, M.; Yar, M.; Smith, J. D.; Crudden, C. M. Org. Lett. 2015, 17, 50.
doi: 10.1021/ol503213z |
| [11] |
Choi, J.; Fu, G. C. J. Am. Chem. Soc. 2012, 134, 9102.
doi: 10.1021/ja303442q |
| [12] |
Kadunce, N. T.; Reisman, S. E. J. Am. Chem. Soc. 2015, 137, 10480.
doi: 10.1021/jacs.5b06466 |
| [13] |
(a) Wheeler, C.; West, K. N.; Liotta, C. L.; Eckert, C. A. Chem. Commun. 2001, 887.
|
|
(b) Kim, D. W.; Song, C. E.; Chi, D. Y. J. Org. Chem. 2003, 68, 4281.
doi: 10.1021/jo034109b |
|
| [14] |
Ratani, T. S.; Bachman, S.; Fu, G. C.; Peters, J. C. J. Am. Chem. Soc. 2015, 137, 13902.
doi: 10.1021/jacs.5b08452 pmid: 26491957 |
| [15] |
Chen, G.; Wang, Z.; Wu, J.; Ding, K. Org. Lett. 2008, 10, 4573.
doi: 10.1021/ol801812a |
| [16] |
Shirsath, S. R.; Shinde, G. H.; Shaikh, A. C.; Muthukrishnan, M. J. Org. Chem. 2018, 83, 12305.
doi: 10.1021/acs.joc.8b01926 pmid: 30203649 |
| [17] |
Liu, S.; Meng, L.; Zeng, X.; Hammond, G. B.; Xu, B. Chin. J. Chem. 2021, 39, 913.
doi: 10.1002/cjoc.202000579 |
| [18] |
(a) Ambrożak, A.; Steinebach, C.; Gardner, E. R.; Beedie, S. L.; Schnakenburg, G.; Figg, W. D.; Gütschow, M. ChemMedChem 2016, 11, 2621.
doi: 10.1002/cmdc.201600496 |
|
(b) Singh, A.; Hakk, H.; Lupton, S. J. J. Labelled Compd. Radiopharm. 2018, 61, 386.
doi: 10.1002/jlcr.3597 |
|
|
(c) Wang, L.-L.; Battini, N.; Bheemanaboina, R. R. Y.; Zhang, S.-L.; Zhou, C.-H. Eur. J. Med. Chem. 2019, 167, 105.
doi: 10.1016/j.ejmech.2019.01.072 |
|
| [19] |
(a) Kiledal, S. A.; Jourdain, R.; Vellalath, S.; Romo, D. Org. Lett. 2021, 23, 6622.
doi: 10.1021/acs.orglett.1c02044 pmid: 35862275 |
|
(b) Cheng, F.; Chen, T.; Huang, Y.-Q.; Li, J.-W.; Zhou, C.; Xiao, X.; Chen, F.-E. Org. Lett. 2022, 24, 115.
doi: 10.1021/acs.orglett.1c03688 pmid: 35862275 |
|
|
(c) Moghadam, F. A.; Hicks, E. F.; Sercel, Z. P.; Cusumano, A. Q.; Bartberger, M. D.; Stoltz, B. M. J. Am. Chem. Soc. 2022, 144, 7983.
doi: 10.1021/jacs.2c02960 pmid: 35862275 |
|
|
(d) Wu, J.; Young, C. M.; Watts, A. A.; Slawin, A. M. Z.; Boyce, G. R.; Bühl, M.; Smith, A. D. Org. Lett. 2022, 24, 4040.
doi: 10.1021/acs.orglett.2c01486 pmid: 35862275 |
|
|
(e) Cochrane, S. R.; Kerr, M. A. Org. Lett. 2022, 24, 5509.
doi: 10.1021/acs.orglett.2c01810 pmid: 35862275 |
|
| [20] |
(a) Ueda, M.; Nishimura, K.; Ryu, I. Synlett 2013, 24, 1683.
doi: 10.1055/s-0033-1339199 |
|
(b) Wu, G.; Xu, S.; Deng, Y.; Wu, C.; Zhao, X.; Ji, W.; Zhang, Y.; Wang, J. Tetrahedron 2016, 72, 8022.
doi: 10.1016/j.tet.2016.10.031 |
|
|
(c) Li, C.; Zhang, Y.; Sun, Q.; Gu, T.; Peng, H.; Tang, W. J. Am. Chem. Soc. 2016, 138, 10774.
doi: 10.1021/jacs.6b06285 |
| [1] | 张勇, 田志高, 黄琳, 侯秋飞, 范红红, 汪万强. α-氰醇甲磺酸酯在合成α-氨基腈类化合物中的应用[J]. 有机化学, 2024, 44(2): 561-572. |
| [2] | 黄志友, 杨平, 何波, 欧文霞, 袁思雨. 吗啉磺酰胺化合物的设计、合成及其抑制大豆萌芽活性的研究[J]. 有机化学, 2024, 44(1): 309-315. |
| [3] | 陈文龙, 李慧敏, 杨鹏飞, 郑东程, 杨高升. 2-芳甲酰基甲亚基丙二酸酯与Corey叶立德的反应[J]. 有机化学, 2023, 43(4): 1472-1482. |
| [4] | 朱源, 陈乐园, 侯文彬, 李祎亮. 亲核氟源参与的芳环氟-18标记反应研究进展[J]. 有机化学, 2021, 41(5): 1774-1788. |
| [5] | 王霞, 屈益欣, 龙城宇, 王雪强. 杂芳基烷基醚、杂芳基卤化物与(氘代)醇的亲核醚化[J]. 有机化学, 2021, 41(2): 795-805. |
| [6] | 李鑫玲, 刘会丽, 张顺吉. 炔丙醇与烯醇硅醚的直接亲核取代反应[J]. 有机化学, 2021, 41(1): 407-411. |
| [7] | 刘娜, 郭思岐, 刘俊芳, 陈彦韬, 徐晓明, 张静, 康亚青, 罗成, 陈示洁, 陈华. 连氨基脲链的三氮唑并噻二唑类DOT1L抑制剂的设计、合成及活性[J]. 有机化学, 2020, 40(8): 2450-2459. |
| [8] | 李若馨, 韩锐, 高锦明. 鸟巢烷二萜关键中间体的合成[J]. 有机化学, 2020, 40(7): 2148-2152. |
| [9] | 赵转霞, 王君姣, 黄丹凤, 杨政, 赵芳霞, 虎永琴, 徐炜刚, 胡雨来. 锡粉促进下3-芳基-3-羟基-2-氧化吲哚的烯丙基化反应研究[J]. 有机化学, 2020, 40(7): 2026-2034. |
| [10] | 张顺吉, 刘会丽. 硫酸催化的炔丙醇快速亲核取代反应[J]. 有机化学, 2020, 40(5): 1257-1265. |
| [11] | 韩满意, 潘虹, 姚紫云, 李琦. 四丁基溴化铵催化的布鲁克重排/烷基化反应[J]. 有机化学, 2020, 40(12): 4274-4283. |
| [12] | 田禾, 吴景卫, 刘钰强, 谢亚非, 王建武, 赵桂龙. 含烷氧基取代的三唑类结构的尿酸转运体1抑制剂的高效合成方法[J]. 有机化学, 2017, 37(7): 1748-1756. |
| [13] | 张小祥, 吕昌, 李萍, 付博, 姚薇薇. Lewis/Brønsted酸催化炔丙醇在亲核取代反应中的研究进展[J]. 有机化学, 2016, 36(6): 1287-1298. |
| [14] | 孙志东, 朱云龙, 黄海洋, 宋贤荣, 肖强. L-胞嘧啶异核苷的全合成研究[J]. 有机化学, 2016, 36(11): 2729-2734. |
| [15] | 张小祥, 孙小萍, 谈继淮, 樊辉, 饶卫东. 烯丙醇在亲核取代反应中的研究进展[J]. 有机化学, 2015, 35(10): 2049-2058. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||