有机化学 ›› 2025, Vol. 45 ›› Issue (1): 286-296.DOI: 10.6023/cjoc202403053 上一篇 下一篇
研究论文
吴律嘉a, 黎江东b, 石忠花a, 金鑫a, 王先恒a, 赵长阔a,*( ), 黄强a,*(
), 黄强a,*( )
)
收稿日期:2024-05-16
修回日期:2024-06-20
发布日期:2024-07-24
基金资助:
Lüjia Wua, Jiangdong Lib, Zhonghua Shia, Xin Jina, Xianheng Wanga, Changkuo Zhaoa( ), Qiang Huanga(
), Qiang Huanga( )
)
Received:2024-05-16
Revised:2024-06-20
Published:2024-07-24
Contact:
*E-mail: Supported by:文章分享

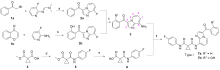
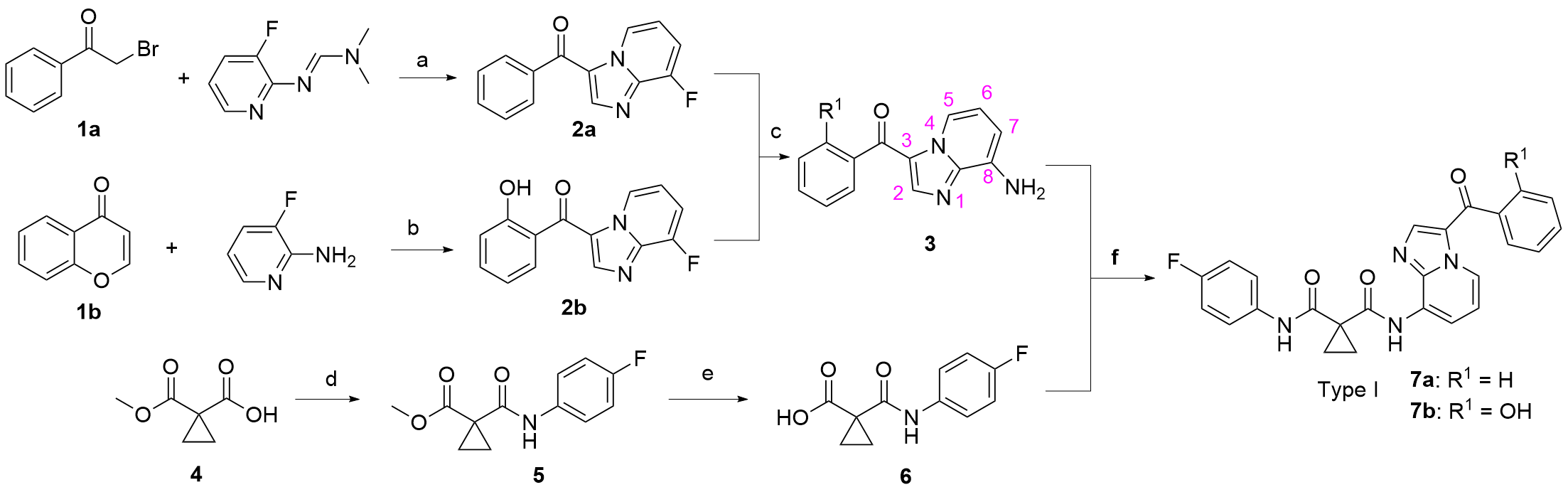
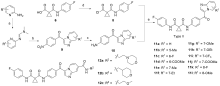
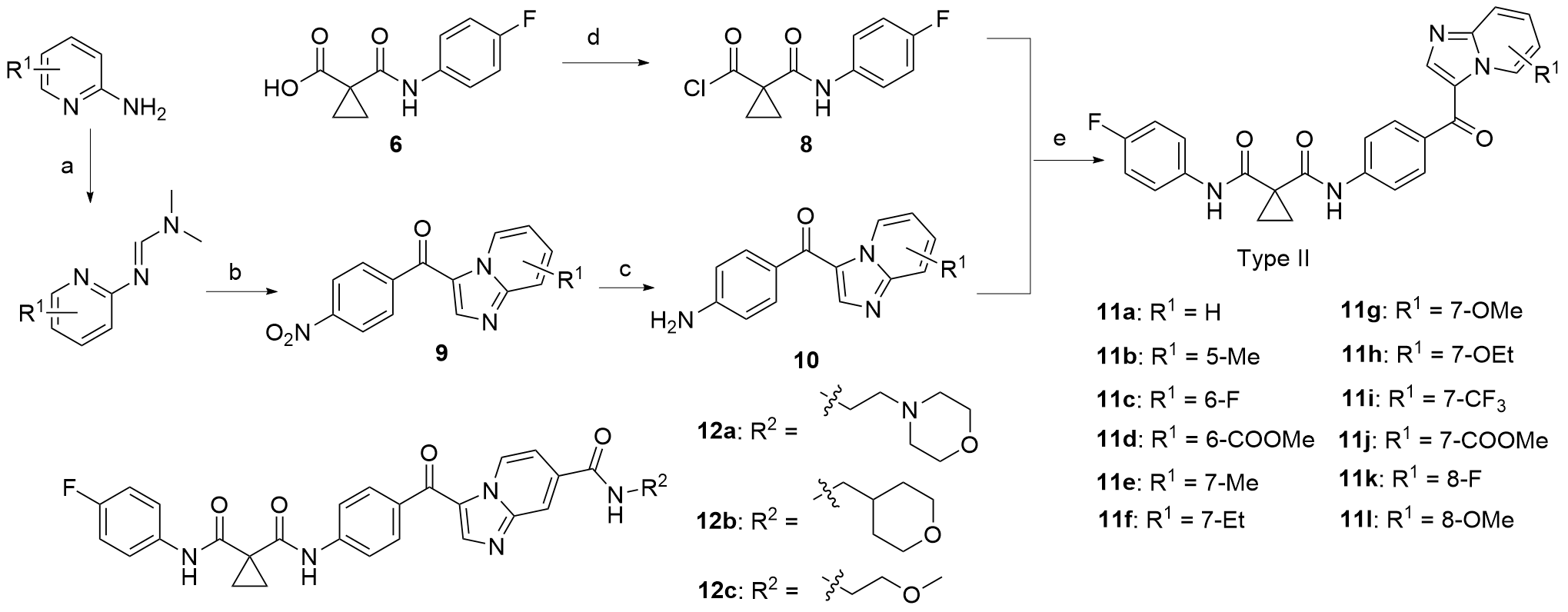
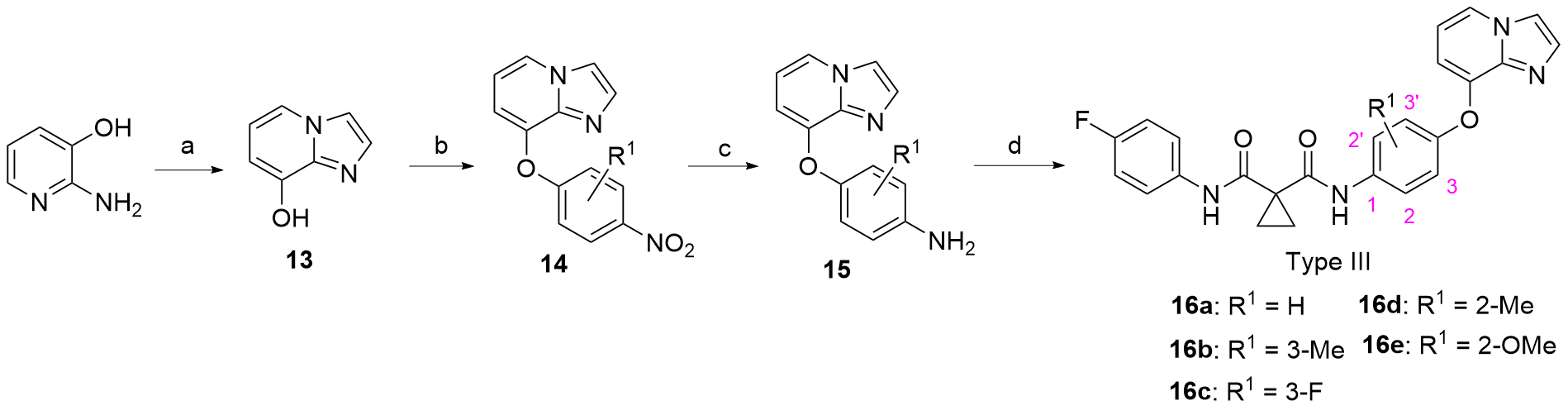
合成了一系列含有咪唑并[1,2-a]吡啶的环丙烷二甲酰胺衍生物, 评估了这些化合物对FLT3-ITD激酶的抑制作用和对表达FLT3-ITD的两种急性髓系白血病细胞株的抗增殖作用. 基于咪唑并[1,2-a]吡啶的不同取代基, 对22种化合物进行了初步的构效关系探索. 结果表明, 大多数化合物对FLT3-ITD激酶具有一定的抑制作用, IC50值均低于0.5 μmol•L-1. 其中, N-(4-氟苯基)-N-(4-(7-((2-吗啉代乙基)氨酰基)咪唑并[1,2-a]吡啶-3-羰基)苯基)环丙烷-1,1-二甲酰胺(12a)具有最佳的FLT3-ITD激酶抑制活性, 且对表达FLT3-ITD的细胞系MV4-11和MOLM-13抗增殖作用最强, IC50值分别为0.06和0.2 μmol•L-1. 此外, 12a对非FLT3突变的细胞系, 如THP-1、HCT-116、A549、HepG2、K562和MCF-7细胞无抗增殖作用, 且对正常人肾小管上皮细胞(HK-2)、人肝祖细胞(HepaRG)和人胚胎肾细胞(HEK293)无细胞毒性. 化合物12a虽然对FLT3-ITD激酶的抑制活性和抗肿瘤细胞增值活性弱于阳性药Cabozantinib, 但为FLT3-ITD抑制剂的进一步研究提供一定的参考.
吴律嘉, 黎江东, 石忠花, 金鑫, 王先恒, 赵长阔, 黄强. 含咪唑并[1,2-a]吡啶的环丙烷二甲酰胺衍生物的合成及其抗急性髓系白血病活性研究[J]. 有机化学, 2025, 45(1): 286-296.
Lüjia Wu, Jiangdong Li, Zhonghua Shi, Xin Jin, Xianheng Wang, Changkuo Zhao, Qiang Huang. Synthesis and Anti-acute Myeloid Leukemia Activity of Cyclopropane-1,1-diamide Derivatives Containing Imidazo[1,2-a]pyridine[J]. Chinese Journal of Organic Chemistry, 2025, 45(1): 286-296.

| Compd. | IC50/(μmol•L-1) | ||
|---|---|---|---|
| FLT3-ITD | MV4-11a | MOLM-13a | |
| 7a | >1.0 | >100 | >100 |
| 7b | >1.0 | 60.3 | 93.3 |
| Cabozantinib | 0.005 | 0.003 | 0.045 |
| Compd. | IC50/(μmol•L-1) | ||
|---|---|---|---|
| FLT3-ITD | MV4-11a | MOLM-13a | |
| 7a | >1.0 | >100 | >100 |
| 7b | >1.0 | 60.3 | 93.3 |
| Cabozantinib | 0.005 | 0.003 | 0.045 |
| Compd. | IC50/(μmol•L-1) | ||
|---|---|---|---|
| FLT3-ITD | MV4-11a | MOLM-13a | |
| 11a | >1.0 | 5.4 | 6.2 |
| 11b | .0642 | 0.6 | 2.0 |
| 11c | >1.0 | 56.1 | >100 |
| 11d | 0.506 | 4.2 | >100 |
| 11e | 0.369 | 2.2 | 1.6 |
| 11f | 0.232 | 0.7 | 0.7 |
| 11g | 0.246 | 5.9 | 0.7 |
| 11h | 0.228 | 0.06 | 2.0 |
| 11i | >1.0 | 9.4 | 4.2 |
| 11j | 0.284 | 37.5 | 26.1 |
| 11k | >1.0 | 68.51 | 44.1 |
| 11l | >1.0 | 72 | >100 |
| Cabozantinib | 0.005 | 0.006 | 0.051 |
| Compd. | IC50/(μmol•L-1) | ||
|---|---|---|---|
| FLT3-ITD | MV4-11a | MOLM-13a | |
| 11a | >1.0 | 5.4 | 6.2 |
| 11b | .0642 | 0.6 | 2.0 |
| 11c | >1.0 | 56.1 | >100 |
| 11d | 0.506 | 4.2 | >100 |
| 11e | 0.369 | 2.2 | 1.6 |
| 11f | 0.232 | 0.7 | 0.7 |
| 11g | 0.246 | 5.9 | 0.7 |
| 11h | 0.228 | 0.06 | 2.0 |
| 11i | >1.0 | 9.4 | 4.2 |
| 11j | 0.284 | 37.5 | 26.1 |
| 11k | >1.0 | 68.51 | 44.1 |
| 11l | >1.0 | 72 | >100 |
| Cabozantinib | 0.005 | 0.006 | 0.051 |
| Compd. | IC50/(μmol•L-1) | ||
|---|---|---|---|
| FLT3-ITD | MV4-11a | MOLM-13a | |
| 12a | 0.127 | 0.06 | 0.2 |
| 12b | 0.322 | 2.40 | 8.0 |
| 12c | 0.0425 | 0.11 | 1.3 |
| Cabozantinib | 0.005 | 0.001 | 0.038 |
| Compd. | IC50/(μmol•L-1) | ||
|---|---|---|---|
| FLT3-ITD | MV4-11a | MOLM-13a | |
| 12a | 0.127 | 0.06 | 0.2 |
| 12b | 0.322 | 2.40 | 8.0 |
| 12c | 0.0425 | 0.11 | 1.3 |
| Cabozantinib | 0.005 | 0.001 | 0.038 |
| Compd. | IC50/(μmol•L-1) | ||
|---|---|---|---|
| FLT3-ITD | MV4-11a | MOLM-13a | |
| 16a | >1.0 | 11.36 | 21.5 |
| 16b | >1.0 | 51.21 | 50.5 |
| 16c | >1.0 | 41.11 | >100 |
| 16d | >1.0 | 78.41 | >100 |
| 16e | >1.0 | >100 | >100 |
| Cabozantinib | 0.005 | 0.008 | 0.041 |
| Compd. | IC50/(μmol•L-1) | ||
|---|---|---|---|
| FLT3-ITD | MV4-11a | MOLM-13a | |
| 16a | >1.0 | 11.36 | 21.5 |
| 16b | >1.0 | 51.21 | 50.5 |
| 16c | >1.0 | 41.11 | >100 |
| 16d | >1.0 | 78.41 | >100 |
| 16e | >1.0 | >100 | >100 |
| Cabozantinib | 0.005 | 0.008 | 0.041 |
| Cell line | IC50/(μmol•L-1) | |
|---|---|---|
| 12a | Cabozantinib | |
| MV4-11 | 0.06 | 0.003 |
| MOLM-13 | 0.2 | 0.045 |
| THP-1 | >100 | 20.3[ |
| HCT-116 | >100 | 15.35[ |
| A549 | >100 | 0.76[ |
| HepG2 | >100 | 8.48[ |
| K562 | >100 | 2.2[ |
| MCF-7 | >100 | 0.45[ |
| HEK-293 | >100 | >100 |
| HepaRG | >100 | — |
| HK-2 | >100 | 1.9 |
| Cell line | IC50/(μmol•L-1) | |
|---|---|---|
| 12a | Cabozantinib | |
| MV4-11 | 0.06 | 0.003 |
| MOLM-13 | 0.2 | 0.045 |
| THP-1 | >100 | 20.3[ |
| HCT-116 | >100 | 15.35[ |
| A549 | >100 | 0.76[ |
| HepG2 | >100 | 8.48[ |
| K562 | >100 | 2.2[ |
| MCF-7 | >100 | 0.45[ |
| HEK-293 | >100 | >100 |
| HepaRG | >100 | — |
| HK-2 | >100 | 1.9 |
| [1] |
(a) Doe, I. S.; Smith, J.; Roe, P. J. Am. Chem. Soc. 1968, 90, 8234.
|
|
(b) Pelcovits, A.; Niroula, R. R. I. Med. J. 2013, 103(3), 38.
|
|
|
(c) Shimony, S.; Stahl, M.; Stone, R. M. Am. J. Hematol. 2023, 98, 502.
|
|
|
(d) Newell, L. F.; Cook, R. J. BMJ [Br. Med. J.] 2021, 375, n2026.
|
|
|
(e) Padmakumar, D.; Chandraprabha, V. R.; Gopinath, P.; Vimala Devi, A. R. T.; Anitha, G. R. J.; Sreelatha, M. M.; Padmakumar, A.; Sreedharan, H. Leuk. Res. 2021, 111, 106727.
|
|
|
(f) Pollyea, D. A.; Altman, J. K.; Assi, R.; Bixby, D.; Fathi, A. T.; Foran, J. M.; Gojo, I.; Hall, A. C.; Jonas, B. A.; Kishtagari, A.; Lancet, J.; Maness, L.; Mangan, J.; Mannis, G.; Marcucci, G.; Mims, A.; Moriarty, K.; Ali, M. M. J. Natl. Compr. Cancer Network 2023, 21, 503.
|
|
| [2] |
(a) Narayanan, D.; Weinberg, O. K. Int. J. Lab. Hematol. 2020, 42, 3.
|
|
(b) Chopra, M.; Bohlander, S. K. Genes, Chromosomes Cancer 2019, 58, 850.
|
|
|
(c) Kwon, A.; Weinberg, O. K. Clin. Lab. Med. 2023, 43, 657.
|
|
|
(d) DiNardo, C. D.; Erba, H. P.; Freeman, S. D.; Wei, A. H. Lancet 2023, 401, 2073.
|
|
| [3] |
(a) Thol, F.; Ganser, A. Curr. Treat. Options Oncol. 2020, 21, 66.
|
|
(b) Ochs, M. A.; Marini, B. L.; Perissinotti, A. J.; Foucar, C. E.; Pettit, K.; Burke, P.; Bixby, D. L.; Benitez, L. L. Ann. Hematol. 2022, 101, 1627.
|
|
|
(c) Bewersdorf, J. P.; Abdel-Wahab, O. Genes Dev. 2022, 36, 259.
|
|
|
(d) Bhansali, R. S.; Pratz, K. W.; Lai, C. J. Hematol. Oncol. 2023, 16, 9.
|
|
|
(e) Choi, J. H.; Shukla, M.; Abdul-Hay, M. Acta Haematol. 2023, 146, 431.
|
|
|
(f) Kayser, S.; Levis, M. J. Br. J. Haematol. 2022, 196, 316.
|
|
|
(g) Khanal, N.; Upadhyay, B. S.; Bhatt, V. R. Clin. Pharmacol. Ther. 2020, 108, 506.
|
|
|
(h) Reville, P. K.; Kadia, T. Curr. Treat. Options Oncol. 2020, 21, 34.
|
|
| [4] |
(a) Kantarjian, H. M.; Kadia, T. M.; DiNardo, C. D.; Welch, M. A.; Ravandi, F. Cancer 2021, 127, 1186.
|
|
(b) Rowe, J. M. Best Pract. Res., lin. Haematol. 2021, 34, 101248.
|
|
| [5] |
Kazi, J. U.; Rönnstrand, L. Physiol. Rev. 2019, 99, 1433.
|
| [6] |
(a) Isidori, A.; Visani, G.; Ferrara, F. Curr. Opin. Oncol. 2023, 35, 589.
|
|
(b) Nitika
|
|
| [7] |
(a) Patnaik, M. M. Leuk. Lymphoma 2018, 59, 2273.
|
|
(b) Grob, T.; Sanders, M. A.; Vonk, C. M.; Kavelaars, F. G.; Rijken, M.; Hanekamp, D. W.; Gradowska, P. L.; Cloos, J.; Fløisand, Y.; Kooy, M.; Manz, M. G.; Ossenkoppele, G. J.; Tick, L. W.; Havelange, V.; Löwenberg, B.; Jongen-Lavrencic, M.; Valk, P. J. M. J. Clin. Oncol. 2023, 41, 756.
|
|
| [8] |
(a) Bystrom, R.; Levis, M. J. Curr. Oncol. Rep. 2023, 25, 369.
|
|
(b) Kiyoi, H.; Kawashima, N.; Ishikawa, Y. Cancer Sci. 2020, 111, 312.
|
|
|
(c) Zhang, Y.; Yuan, L. Sci. Rep. 2021, 11, 13236.
|
|
|
(d) Lv, K.; Ren, J. G.; Han, X.; Gui, J.; Gong, C.; Tong, W. Blood 2021, 138, 2244.
|
|
| [9] |
(a) Zhong, Y.; Qiu, R. Z.; Sun, S. L.; Zhao, C.; Fan, T. Y.; Chen, M.; Li, N. G.; Shi, Z. H. J. Med. Chem. 2020, 63, 12403.
|
|
(b) Daver, N.; Venugopal, S.; Ravandi, F. Blood Cancer J. 2021, 11, 104.
|
|
|
(c) Zhao, J. C.; Agarwal, S.; Ahmad, H.; Amin, K.; Bewersdorf, J. P.; Zeidan, A. M. Blood Rev. 2022, 52, 100905.
|
|
|
(d) Egbuna, C.; Patrick-Iwuanyanwu, K. C.; Onyeike, E. N.; Khan, J.; Alshehri, B. J. Biomol. Struct. Dyn. 2022, 40, 12248.
|
|
|
(e) Arai, Y.; Chi, S.; Minami, Y.; Yanada, M. Int. J. Hematol. 2022, 116, 351.
|
|
| [10] |
(a) Antar, A. I.; Otrock, Z. K.; Jabbour, E.; Mohty, M.; Bazarbachi, A. Leukemia 2020, 34, 682.
|
|
(b) Garciaz, S.; Hospital, M. A. OncoTargets Ther. 2023, 16, 31.
|
|
|
(c) O'Farrell, A. C.; Miller, I. S.; Evans, R.; Alamanou, M.; Cary, M.; Udupi, G. M.; Lafferty, A.; Monsefi, N.; Cremona, M.; Prehn, J. H. M.; Verheul, H. M.; Gallagher, W. M.; Gehrmann, M.; Byrne, A. T. Proteomics: Clin. Appl. 2019, 13, e1800159.
|
|
|
(d) Cerchione, C.; Raíndo, A. P.; Orgueira, A. M.; Torre, A. M.; Pérez, L. B.; Marconi, G.; Isidori, A.; Encinas, M. M.; Martinelli, G. Expert Rev. Hematol. 2021, 14, 851.
|
|
| [11] |
(a) Reed, D. R.; Sen, J. M.; Pierce, E. J.; Elsarrag, R. Z.; Keng, M. K. J. Oncol. Pharm. Pract. 2020, 26, 1200.
|
|
(b) Naqvi, K.; Ravandi, F. Leuk. Lymphoma 2019, 60, 1866.
|
|
|
(c) Cortes, J. Clin. Adv. Hematol. Oncol. 2023, 21, 240.
|
|
|
(d) Zhao, J.; Song, Y.; Liu, D. Biomark. Res. 2019, 7, 19.
|
|
|
(e) Wang, E. S.; Goldberg, A. D.; Tallman, M.; Walter, R. B.; Karanes, C.; Sandhu, K.; Vigil, C. E.; Collins, R.; Jain, V.; Stone, R. M. J. Clin. Oncol. 2024, 42, 1776.
|
|
|
(f) Levis, M.; Perl, A. E. Blood Adv. 2020, 4, 1178.
|
|
| [12] |
(a) Ferng, T. T.; Terada, D.; Ando, M.; Tarver, T. C.; Chaudhary, F.; Lin, K. C.; Logan, A. C.; Smith, C. C. Mol. Cancer Ther. 2022, 21, 844.
|
|
(b) Ferng, T. T.; Tarver, T. C.; Smith, C. C. Blood 2017, 130, 2632.
|
|
|
(c) Leyte-Vidal, A.; Phan, S.; Khare, P.; Hu, F.; Zhang, C.; Cao, P.; Shah, N. P. Blood 2023, 142, 2265.
|
|
|
(d) Xu, J.; Ong, E. H. Q.; Hill, J.; Chen, A.; Chai, C. L. L. Bioorg. Med. Chem. 2014, 22, 6625.
|
|
|
(e) Law, B.; Rughwani, T.; Archer, T. C.; Kumar, L.; Lu, D.; Somanath, P.; Ma, Y.; Wang, X.; Sperandio, D.; Morris, S. Blood 2022, 140, 6191.
|
|
| [13] |
Friedman, R. Biochim. Biophys. Acta, Rev. Cancer 2022, 1877, 188666.
|
| [14] |
(a) Pragyandipta, P.; Pedapati, R. K.; Reddy, P. K.; Nayek, A.; Meher, R. K.; Guru, S. K.; Kantevari, S.; Naik, P. K. Chem.-Biol. Interact. 2023, 382, 110606.
|
|
(b) Hussain, R.; Rehman, W.; Rahim, F.; Khan, S.; Taha, M.; Khan, Y.; Sardar, A.; Khan, I.; Shah, S. A. A. J. Mol. Struct. 2023, 1293, 136185.
|
|
|
(c) Vanam, N. R.; Anireddy, J. S. Chem. Data Collect. 2023, 48, 101092.
|
|
|
(d) Nguyen, W.; Jacobson, J.; Jarman, K. E.; Blackmore, T. R.; Sabroux, H. J.; Lewin, S. R.; Purcell, D. F.; Sleebs, B. E. Eur. J. Med. Chem. 2020, 195, 112254.
|
|
|
(e) Vanda, D.; Zajdel, P.; Soural, M. Eur. J. Med. Chem. 2019, 181, 111569.
|
|
|
(f) Shi, L.; Li, T.; Mei, G.-J. Green Synth. Catal. 2022, 3, 227.
|
|
| [15] |
(a) Dokla, E. M. E.; Abdel-Aziz, A. K.; Milik, S. N.; McPhillie, M. J.; Minucci, S.; Abouzid, K. A. M. Bioorg. Med. Chem. 2022, 56, 116596.
|
|
(b) Peytam, F.; Emamgholipour, Z.; Mousavi, A.; Moradi, M.; Foroumadi, R.; Firoozpour, L.; Divsalar, F.; Safavi, M.; Foroumadi, A. Bioorg. Chem. 2023, 140, 106831.
|
|
|
(c) Boddiboyena, R.; Reddy, G. N.; Seelam, N. V.; Sarma, M.; Kolli, D.; Rajeswari, M.; Gudisela, M. R. Chem. Data Collect. 2023, 46, 101036.
|
|
|
(d) Güçlü, D.; Kuzu, B.; Tozlu, İ.; Taşpınar, F.; Canpınar, H.; Taşpınar, M.; Menges, N. Bioorg. Med. Chem. Lett. 2018, 28, 2647.
|
|
|
(e) Song, Y. N.; Lin, X.; Kang, D.; Li, X.; Zhan, P.; Liu, X.; Zhang, Q. Eur. J. Med. Chem. 2014, 82, 293.
|
|
|
(f) Frett, B.; McConnell, N.; Smith, C. C.; Wang, Y.; Shah, N. P.; Li, H.-Y. Eur. J. Med. Chem. 2015, 94, 123.
|
|
| [16] |
Huang, Q.; Wu, L.; Shi, J.; Li, J.; Lu, W.; Tang, F.; Zhu, L.; Zhong, W.; Zhao, C. Synthesis 2023, 55, 2570.
|
| [17] |
Grüllich, C. Recent Results Cancer Res. 2018, 211, 67.
|
| [18] |
(a) Zhang, J.; Chen, P.; Duan, Y.; Xiong, H.; Li, H.; Zeng, Y.; Liang, G.; Tang, Q.; Wu, D. Eur. J. Med. Chem. 2021, 215, 113273.
|
|
(b) Wang, L. X.; Liu, X.; Xu, S.; Tang, Q.; Duan, Y.; Xiao, Z.; Zhi, J.; Jiang, L.; Zheng, P.; Zhu, W. Eur. J. Med. Chem. 2017, 141, 538.
|
|
| [19] |
Lu, J. W.; Wang, A. N.; Liao, H. A.; Chen, C. Y.; Hou, H. A.; Hu, C. Y.; Tien, H. F.; Ou, D. L.; Lin, L. I. Cancer Lett. 2016, 376, 218.
|
| [20] |
Hassan, A.; Mubarak, F. A. F.; Shehadi, I. A.; Mosallam, A. M.; Temairk, H.; Badr, M.; Abdelmonsef, A. H. J. Enzyme Inhib. Med. Chem. 2023, 38, 2189578
|
| [21] |
Huang, D.; Huang, L.; Zhang, Q.; Li, J. Eur. J. Med. Chem. 2017, 140, 212.
|
| [1] | 秦丽清, 林桂汕, 段文贵, 崔玉成, 杨卯芳, 李芳耀, 李典鹏. 新型长叶烯基萘满并N-酰基吡唑化合物的合成、抗增殖活性、三维定量构效关系及分子对接研究[J]. 有机化学, 2024, 44(6): 1967-1977. |
| [2] | 王妮, 郑姿君, 贾小苹, 赵梦圆, 王亚蕾, 周臣, 王志佳, 肖泽霖, 刘宏民, 可钰. 济源冬凌草甲素衍生物作为潜在抗肿瘤药物的合成及药理学活性研究[J]. 有机化学, 2023, 43(2): 646-659. |
| [3] | 罗云, 高谕康, 燕鹏程, 朱伟明. 浅蓝霉素H的发酵优化与浅蓝苷K的化学合成[J]. 有机化学, 2022, 42(9): 2840-2849. |
| [4] | 高潮, 司晓杰, 池玲玲, 王浩, 戴洪林, 刘丽敏, 汪正捷, 张洋, 王涛, 周耀传, 郑甲信, 可钰, 刘宏民, 张秋荣. 含苯甲醚结构的2,4,5,6-四取代嘧啶衍生物的合成及抗增殖活性研究[J]. 有机化学, 2022, 42(6): 1677-1686. |
| [5] | 张涛, 卫海沅, 马雯, 李张媛, 胡盼盼, 周楠茜, 贺建超, 李婷, 苏明明, 白素平. 新型蓝萼甲素-1,2,3-三氮唑类衍生物的合成与抗增殖活性研究[J]. 有机化学, 2022, 42(11): 3668-3683. |
| [6] | 高潮, 张玉桐, 池玲玲, 王浩, 马家婕, 毕梦鑫, 戴洪林, 司晓杰, 刘丽敏, 张洋, 郑甲信, 可钰, 刘宏民, 张秋荣. 新型2,4,6-三取代嘧啶衍生物的合成及抗增殖活性评价[J]. 有机化学, 2022, 42(11): 3824-3834. |
| [7] | 张路野, 张洋, 包崇男, 杨鹏, 李二冬, 孟娅琪, 崔飞, 周蕊, 黄诗雨, 郑甲信, 单丽红, 刘宏民, 张秋荣. 新型1,3-二取代酞嗪酮类衍生物的合成及抗肿瘤活性研究[J]. 有机化学, 2020, 40(3): 794-800. |
| [8] | 孙海燕, 孙宏顺, 刘明珍, 黄伟, 杨光富. 基于二硫代氨基甲酸酯活性亚结构的先导优化及其抗增殖活性[J]. 有机化学, 2018, 38(8): 2067-2075. |
| [9] | 张娅玲, 马沙沙, 李夏冰, 侯巧丽, 吕梦娇, 郝云霞, 王伟, 李宝林. 吡咯并三嗪衍生物的合成及其对肿瘤细胞增殖的抑制活性[J]. 有机化学, 2018, 38(12): 3270-3277. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||