

分子内氧化偶联反应在合成复杂吲哚生物碱骨架中的应用
收稿日期: 2013-01-15
修回日期: 2013-01-31
网络出版日期: 2013-02-01
基金资助
国家重点基础研究发展计划(973计划, No. 2010CB833200)资助项目.
Intramolecular Oxidative Coupling: Applications in Synthesis of Complex Indole Akaloid Scaffolds
Received date: 2013-01-15
Revised date: 2013-01-31
Online published: 2013-02-01
Supported by
Project supported by the National Basic Research Program of China (973 Program, No. 2010CB833200).
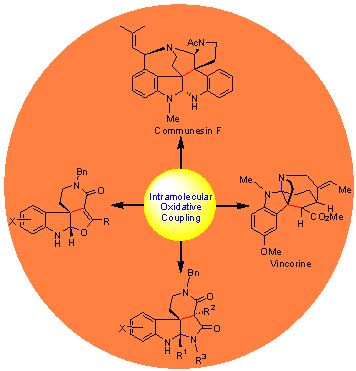
通过含有吲哚底物的分子内氧化偶联反应, 成功地构建了Communesin家族生物碱的螺吲哚啉季碳中心, 从而完成了(-)-Communesins A, B和F的对映选择性合成. 接下来我们发展了分子内氧化偶联/缩合串联反应策略, 得到了天然产物(-)-Vincorine的核心四环骨架, 然后再经过五步转化完成了Vincorine的全合成. 从药物化学角度来看, 分子内氧化偶联/缩合串联提供了一个快速方便地合成含有多环吲哚啉骨架的方法. 采用相同的串联反应策略, 我们分别从色胺衍生的β-酮酸酰胺和丙二酸二酰胺出发, 一步构建了多环螺吲哚啉和多环吲哚啉并吡咯环骨架分子.
谢卫青 , 左智伟 , 资伟伟 , 马大为 . 分子内氧化偶联反应在合成复杂吲哚生物碱骨架中的应用[J]. 有机化学, 2013 , 33(05) : 869 -876 . DOI: 10.6023/cjoc201301035
Intramolecular oxidative coupling of tryptamine incorporated amide was used to create the quaternary spiroindoline carbon center of communesins, which enabled a short asymmetric synthesis of (-)-communesins A and B and F. The fused tetra-ring framework of Vincorine was established by an intramolecular oxidative coupling/condensative cyclization process, which was further advanced to (-)-vincorine in 5 steps. From a medicinal standpoint, such a cascade process provides a highly diverse, efficient method for the construction of polycyclic spiroindoline scaffolds. Starting from easily accessible tryptamine incorporated β-ketoamides and malonamides, polyclic spiroindolines and pyrroloindolines could be directly obtained by adopting the same cascade strategy.

[1] Selected examples for oxidative homo-coupling: (a) Ivanoff, D.; Spassoff, A. Bull. Soc. Chim. Fr. 1935, 2, 76.
(b) Rathke, M. W.; Lindert, A. J. Am. Chem. Soc. 1971, 93, 4605.
(c) Brocksom, T. J.; Petragnani, N.; Rodrigues, R.; La Scala Teixeira, H. Synthesis 1975, 396.
(d) Frazier, R. H.; Harlow, R. L. J. Org. Chem. 1980, 45, 5408.
(e) Belletire, J. L.; Fry, D. F. J. Org. Chem. 1987, 45, 2549.
(f) Renaud, P.; Fox, M. A. J. Org. Chem. 1988, 45, 3745.
(g) Quermann, R.; Maletz, R.; Schafer, H. J. Liegigs Ann. Chem. 1993, 11, 1219.
(h) Kise, N.; Tokioka, K.; Aoyama, Y. J. Org. Chem. 1995, 60, 1100.
(i) Langer, T.; Illich, M.; Felmchen, G. Tetrahedron Lett. 1995, 36, 4409.
(j) Kim, J. W.; Lee, J. J.; Lee, S.-H.; Ahn, K.-H. Synth. Commun. 1998, 28, 1287.
[2] Selected examples for oxidative hetero-coupling: (a) Ito, Y.; Konoike, T.; Harada, T.; Saegusa, T. J. Am. Chem. Soc. 1977, 99, 1487.
(b) Baran, P. S.; Richter, J. M.; Lin, D. W. Angew. Chem., Int. Ed. 2005, 44, 609.
(c) Baran, P. S.; DeMartino, M. P. Angew. Chem., Int. Ed. 2006, 45, 7083.
(d) Richter, J. M.; Whitefield, B.; Maimone, T. J.; Lin, D. W.; Castroviejo, P.; Baran, P. S. J. Am. Chem. Soc. 2007, 129, 12857.
(e) Richter, J. M.; Whitefield, B.; Maimone, T. J.; Lin, D. W.; Castroviejo, P.; Baran, P. S. J. Am. Chem. Soc. 2007, 129, 12857.
(f) DeMartino M. P.; Chen, K.; Baran, P. S. J. Am. Chem. Soc. 2008, 130, 11546.
[3] (a) Baran, P. S.; Richter, J. M. J. Am. Chem. Soc. 2004, 126, 7450.
(b) Baran, P. S.; Richter, J. M. J. Am. Chem. Soc. 2005, 127, 15394.
(c) Richter, J. M.; Whitefield, B.; Maimone, T. J.; Lin, D. W.; Castroviejo, P.; Baran, P. S. J. Am. Chem. Soc. 2007, 129, 12857.
(d) Baran, P. S.; Maimone, T. J.; Richter, J. M. Nature 2007, 446, 404.
(e) Richter, J. M.; Ishihara, Y.; Masuda, T.; Whitefield, B. W.; Llamas, T.; Pohjakallio, A.; Baran, P. S. J. Am. Chem. Soc. 2008, 130, 17938.
[4] (a) Baran, P. S.; Richter, J. M. J. Am. Chem. Soc. 2004, 126, 7450.
(b) Baran, P. S.; Richter, J. M. J. Am. Chem. Soc. 2005, 127, 15394.
(c) Baran, P. S.; Maimone, T. J.; Richter, J. M. Nature 2007, 446, 404.
(d) Richter, J. M.; Ishihara, Y.; Masuda, T.; Whitefield, B. W.; Llamas, T.; Pohjakallio, A.; Baran, P. S. J. Am. Chem. Soc. 2008, 130, 17938.
[5] (a) Martin, C. L.; Overman, L. E.; Rohde, J. A. J. Am. Chem. Soc. 2010, 132, 4894.
(b) Martin, C. L.; Overman, L. E.; Rohde, J. A. J. Am. Chem. Soc. 2008, 130, 7568.
Fore review see: (c) Guo, F.; Clift, M. D.; Thomson, R. J. Eur. J. Org. Chem. 2012, 26, 4881.
For other examples see: (d) Li, Q.; Fan, A.; Lu, Z.; Cui, Y.; Lin, W.; Jia, Y. Org. Lett. 2010, 12, 4066.
(e) Li, Q.; Jiang, J.; Fan, A.; Cui, Y.; Jia, Y. Org. Lett. 2011, 13, 312.
[6] (a) Zuo, Z.; Xie, W.; Ma, D. J. Am. Chem. Soc. 2010, 132, 13226.
(b) Zuo, Z.; Ma, D. Angew. Chem., Int. Ed. 2011, 50, 12008.
[7] Zi, W.; Xie, W.; Ma, D. J. Am. Chem. Soc. 2012, 132, 9126.
[8] Fan, F.; Xie, W.; Ma, D. Org. Lett. 2012, 14, 1405.
[9] Fan, F.; Xie, W.; Ma, D. Chem. Commun. 2012, 48, 7571.
[10] (a) Numata, C.; Takahashi, Y.; Ito, T.; Takada, K.; Kawai, Y.; Usami, E.; Matsumura, M.; Imachi, T.; Ito, T.; Hasegawa, T. Tetrahedron Lett. 1993, 34, 2355.
(b) Jadulco, R.; Edrada, R. A.; Ebel, R.; Berg, A.; Schauman, K.; Wray, V.; Steube, K.; Proksch, P. J. Nat. Prod. 2004, 67, 78.
(c) Hayashi, H.; Matsumoto, H.; Akiyama, K. Biosci. Biotechnol. Biochem. 2004, 68, 753.
(d) Dalsgaard, P. W.; Blunt, J. W.; Munro, M. H. G.; Frisvad, J. C.; Christophersen, C. J. Nat. Prod. 2005, 68, 258.
[11] For synthetic studies see: (a) May, J. A.; Stoltz, B. M. Tetrahedron 2006, 62, 5262.
(b) Crawley, S. L.; Funk, R. L. Org. Lett. 2006, 8, 3995.
(c) George, J. H.; Adlington, R. M. Synlett 2008, 14, 2093.
For total syntheses see Refs. [6] and (d) Yang, J.; Wu, H.; Shen, L.; Qin, Y. J. Am. Chem. Soc. 2007, 129, 13794.
(e) Liu, P.; Seo, J. H.; Weinreb, S. M. Angew. Chem. 2010, 122, 2044; Angew. Chem., Int. Ed. 2010, 49, 2000.
(f) Belmar, J.; Funk, R. L. J. Am. Chem. Soc. 2012, 134, 16941.
[12] (a) Smith, G. F. Chem. Ind. 1961, 1120.
(b) Wenkert, E.; Wickberg, B. J. Am. Chem. Soc. 1965, 87, 1580.
For an overview of the akuammiline alkaloid family, see: (c) Ramirez, A.; Garcia-Rubio, S. Curr. Med. Chem. 2003, 10, 1891.
[13] (a) Mokry, J.; Dubravkova, L.; Sefcovic, P. Experientia 1962, 18, 564.
(b) Das, B. C.; Cosson, J. P.; Lukacs, G.; Potier, P. Tetrahedron Lett. 1974, 15, 4229.
(c) Mamatas-Kalamaras, S.; Sevenet, T.; Thal, C.; Potier, P. Phytochemistry 1975, 14, 1637.
[14] Subramaniam, G.; Hiraku, O.; Hayashi, M.; Koyano, T.; Komiyama, K.; Kam, T. S. J. Nat. Prod. 2007, 70, 1783.
[15] (a) Schnoes, H.; Biemann, K.; Mokry, J.; Kompis, I.; Chatterjee, A.; Ganguli, G. J. Org. Chem. 1966, 31, 1641.
(b) Ahmad, Y.; Fatima, K.; Rahman, A.; Occolowitz, J.; Solheim, B.; Clardy, J.; Garnick, R.; Le Quesne, P. J. Am. Chem. Soc. 1977, 99, 1943.
[16] Cai, X. H.; Tan, Q. G.; Liu, Y. P.; Feng, T.; Du, Z. Z.; Li, W. Q.; Luo, X. D. Org. Lett. 2008, 10, 577.
[17] (a) Dounay, D. B.; Vollhardt, K. P. C. J. Am. Chem. Soc. 1990, 112, 5653.
(b) Lévy, J.; Sapi, J.; Laronze, J. Y.; Royer, D.; Toupet, L. Synlett 1992, 7, 601.
(c) Dounay, A. B.; Overman, L. E.; Wrobleski, A. D. J. Am. Chem. Soc. 2005, 127, 10186.
(d) Shen, L.; Zhang, M.; Wu, Y.; Qin, Y. Angew. Chem., Int. Ed. 2008, 47, 3618.
(e) Jones, S. B.; Simons, B.; Macmillan, D. W. C. J. Am. Chem. Soc. 2009, 131, 13606.
(f) Yasui, Y.; Kinugawab, T.; Takemoto, Y. Chem. Commun. 2009, 28, 4275.
(g) Zhang, M.; Huang, X.; Shen, L.; Qin, Y. J. Am. Chem. Soc. 2009, 131, 6013.
(h) Zhang, D.; Song, H.; Qin, Y. Acc. Chem. Res. 2011, 44, 447.
(i) Zu, L.; Boal, B. W.; Garg, N. K. J. Am. Chem. Soc. 2011, 133, 8877.
(j) Adams, G. L.; Caroll, P. J.; Smith, A. B. J. Am. Chem. Soc. 2012, 134, 4037.
[18] For reviews, see: (a) Anthoni, U.; Christophersen, C.; Nielsen, P. H. In Alkaloids: Chemical and Biological Perspectives, Vol. 13, Ed.: Pelletier, S. W., Pergamon, New York, 1999, p. 163.
(b) Cordell, G. A.; Saxton, J. E. In the Alkaloids: Chemistry and Physiology, Vol. 20, Eds.: Manske, R. H. F.; Rodrigo, R. G. A., Academic Press, New York, 1981, p. 3.
(c) Hino, T.; Nakagawa, M. In the Alkaloids: Chemistry and Pharmacology, Vol. 34, Ed.: Brossi, A., Academic Press, New York, 1989, p. 1.
(d) Sévenet, T.; Pusset, J. In the Alkaloids: Chemistry and Pharmacology, Vol. 48, Ed.: Cordell, G. A., Academic Press, New York, 1996, p. 1.
(e) Steven, A.; Overman, L. E. Angew. Chem., Int. Ed. 2007, 46, 5488.
(f) Ruiz-Sanchis, P.; Savina, S. A.; Albericio, F.; Álvarez, M. Chem. Eur. J. 2011, 17, 1388.
/
| 〈 |
|
〉 |