

白藜芦醇衍生物的合成及抑制宫颈癌HeLa细胞肿瘤活性
收稿日期: 2013-03-23
修回日期: 2013-04-26
网络出版日期: 2013-05-06
基金资助
上海市教委科研创新重点(No.12ZZ188);上海市科委(No.11430502500);上海高校特聘教授(东方学者)岗位计划(No.405ZK110060002)资助项目.
Synthesis and Anti-tumor Activities of Resveratrol Derivatives on Cervical Cancer HeLa Cells
Received date: 2013-03-23
Revised date: 2013-04-26
Online published: 2013-05-06
Supported by
Project Supported by the Innovation Program of Shanghai Municipal Education Commission (No.12ZZ188), the Science and Technology Commission of Shanghai Municipal (No.11430502500), the Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (No.405ZK110060002).
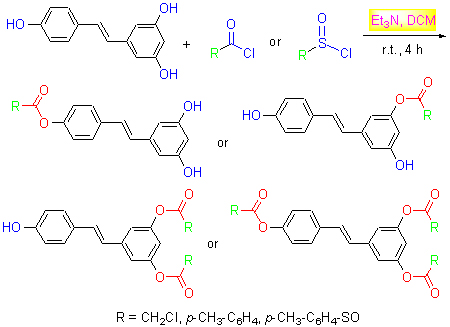
以白藜芦醇、氯乙酰氯、对甲苯磺酰氯、对甲苯甲酰氯为原料, 合成9种新的白藜芦醇衍生物, 其结构经IR, 1H NMR, 13C NMR和HRMS所表征.用3-(4,5-二甲基噻唑-2)-2,5-二苯基四氮唑溴盐(MTT)法测试了目标化合物抑制宫颈癌HeLa细胞的肿瘤活性, 结果表明: 化合物4a, 6a, 7b, 8b和11c对HeLa细胞的抑制活性比白藜芦醇高, 其中化合物6a和8b的抑制效果最明显, 其IC50值分别为22.7和18.0 μmol/L, 活性高于白藜芦醇(IC50=114 μmol/L), 且在150 μmol/L浓度下对HeLa细胞的抑制率达95.5%和87.7%.
杜成 , 任玉杰 , 王庆伟 , 金鹭 . 白藜芦醇衍生物的合成及抑制宫颈癌HeLa细胞肿瘤活性[J]. 有机化学, 2013 , 33(06) : 1279 -1283 . DOI: 10.6023/cjoc201303035
Nine novel resveratrol derivatives were synthesized from resveratrol, chloroacetyl chloride, p-toluenesulfonyl chloride and p-methyl benzoyl chloride.These structures were confirmed by IR, 1H NMR, 13C NMR and HR-MS techniques.The target compounds were evaluated for their anti-tumor activities against cervical cancer HeLa cells by 3-(4,5-dimethylthi- azol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method, and the results indicated that compounds 4a, 6a, 7b, 8b and 11c of all the derivatives displayed better activities than resveratrol against HeLa cells, the compounds 6a and 8b displayed excellent inhibition effect, with IC50 values of 22.7 and 18.0 μmol/L, respectively, even higher than that of resveratrol (IC50=114 μmol/L).The inhibitory ratio of compounds 6a and 8b were 95.5% and 87.7% against HeLa cells at the concentration of 150 μmol/L.

Key words: resveratrol; derivative; synthesis; HeLa cell; anti-tumor activities
/
| 〈 |
|
〉 |