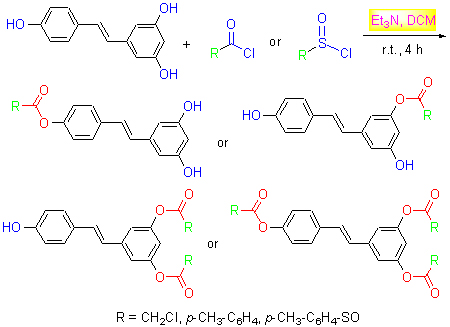
| [1] Pan, M.H.; Lin, C.L.; Tsai, J.H.; Ho, C.T.; Chen, W.J.J.Agric.Food Chem.2010, 58, 226.[2] Juan, M.E.; Wenzel, U.; Daniel, H.; Planas, J.M.J.Agric.Food Chem.2008, 56, 4813.[3] Kundu, J.K.; Surh, Y.J.Cancer Lett.2008, 269, 243.[4] Selvaraj, S.; Mohan, A.; Narayanan, S.; Sethuraman, S.; Krishnan, U.M.J.Med.Chem.2013, 56, 970.[5] Agrawal, M.; Kumar, V.; Singh, A.K.; Kashyap, M.P.; Khanna, V.K.; Siddiqui, M.A.; Pant, A.B.ACS Chem.Neurosci.2013, 4, 285.[6] Udenigwe, C.C.; Ramprasath, V.R.; Aluko, R.E.; Jones, P.J.H.Nutr.Rev.2008, 66, 445.[7] Li, L.; Henry, G.E.; Seeram, N.P.J.Agric.Food Chem.2009, 57, 7282.[8] Yap, S.; Qin, C.X.; Woodman, O.L.Biofactors 2010, 36, 350.[9] Albani, D.; Polito, L.; Signorini, A.; Forloni, G.Biofactors 2010, 36, 370.[10] Bishayee, A.; Darvesh, A.S.; Politis, T.; McGory, R.Liver Int.2010, 30, 1103.[11] Joseph, J.A.Fisher D.R.; Cheng, V.; Rimando, A.M.; Shukitt-Hale, B.J.Agric.Food Chem.2008, 56, 10544.[12] Mouchiroud, L.; Molin, L.; Dallière, N.; Solari, F.Biofactors 2010, 36, 377.[13] Chen, W.P.; Hung, L.M.; Hsueh, C.H.; Lai, L.P.; Su, M.J.Br.J.Pharmacol.2009, 157, 381.[14] Zhu, Z.Q.; Zhang, X.Y.; Chen, J.W.; Xia, J.; Zhai, W.G.; Hu, T.X.J.East China Normal Univ.(Nat.Sci) 2002, 2, 98 (in Chinese).(朱振勤, 张小轶, 陈季武, 夏晶, 翟万垠, 胡天喜, 华东师范大学学报(自然科学版), 2002, 2, 98.)[15] Liu, S.J.; Hu, Y.L.; Wang, X.L.; Zhong, J.; Lin, Z.P.J.Agric.Food Chem.2006, 54, 8082.[16] Reddy, M.A.; Jain, N.; Yada, D.; Kishore, C.; Vangala, J.R.; P.Surendra R.; Addlagatta, A.; Kalivendi, S.V.; Sreedhar, B.J.Med.Chem.2011, 54, 6751.[17] Lv, Y.J.; Zhou, N.; Meng, Q.G.J.Int.Pharm.Res.2008, 35, 377.(in Chinese).(吕玉健, 周宁, 孟庆国, 国际药学杂志, 2008, 35, 377.)[18] Liu, H.C.; Dong, A.J.; Gao, C.M.; Jiang, Y.Y.Prog.Chem.2009, 21, 1500 (in Chinese).(刘华臣, 董爱君, 高春梅, 蒋宇扬, 化学进展, 2009, 21, 1500.) |