

CuI催化邻溴三氟乙酰基苯胺和端炔合成吲哚偶联反应机理的理论研究
收稿日期: 2013-03-08
修回日期: 2013-05-03
网络出版日期: 2013-06-21
基金资助
四川教育厅(No. 13ZA0150)资助项目
Investigation on Coupling Reaction Mechanism from N-(2-Bromophenyl)-2,2,2-trifluoroacetamide and Terminal Alkyne to Indol Catalyzed by CuI
Received date: 2013-03-08
Revised date: 2013-05-03
Online published: 2013-06-21
Supported by
Project supported by the Department of Education of Sichuan Province (No. 13ZA0150).
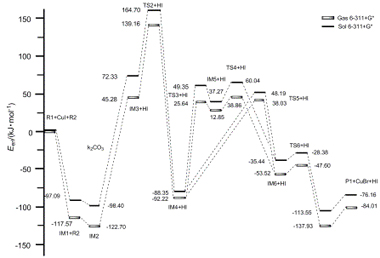
采用密度泛函理论的B3LYP方法对CuI催化邻溴三氟乙酰基苯胺和端炔合成吲哚的偶联反应机理进行了研究. 在6-31+G*基组水平上对反应过程中的所有反应物、过渡态、中间体和产物进行了优化, 通过能量分析及振动频率计算证实了中间体和过渡态的合理性. 在相同基组水平上应用自然键轨道(NBO)理论和分子中的原子(AIM)理论分析了反应过程中反应物的成键特征和轨道间的相互作用, 得到两条可能的反应通道IA和IB, 结果发现反应通道IA为主要反应通道. 同时应用前线轨道理论解释了催化剂作用机制. 为了提高计算精度, 在6-311+G*基组水平上计算了反应机理中所有物质在气相及溶剂化下的单点能, 结果得到与6-31+G*基组计算相同的结论,从理论上说明了催化剂的有效性.
张明 , 王玲玲 , 李来才 , 田安民 . CuI催化邻溴三氟乙酰基苯胺和端炔合成吲哚偶联反应机理的理论研究[J]. 有机化学, 2013 , 33(10) : 2169 -2177 . DOI: 10.6023/cjoc201303011
The reaction mechanism of N-(2-bromophenyl)-2,2,2-trifluoroacetamide and terminal alkyne catalyzed by CuI has been investigated by using density functional theory. The geometries of the reactants, transition states, intermediates and products have been optimized completely at B3LY P/6-31+G* level with the validation of the vibration analysis and the energy calculation. Atoms in molecules (AIM) theories and nature bond orbital (NBO) have been applied to discuss the orbits interaction and the bond natures. Catalytic mechanism is also interpreted by frontier orbital theory. Two possible reaction paths IA and IB were obtained. The results indicated that IA is the really main possible path of the reaction. Meanwhile, the single point energy of the reaction process in gas and solvent at 6-311+G* level has been individually investigated with higher precision. The results indicate that the reaction mechanism and the change trend of correspondence energy at two different levels are consistent. The final result of the our theory study agrees with the experimental data, and it illustrate that CuI is an effective catalyst in this reaction.

/
| 〈 |
|
〉 |