

金属催化芳基硼酸与酮不对称1,2-加成反应研究进展
收稿日期: 2013-05-27
修回日期: 2013-06-24
网络出版日期: 2013-07-03
基金资助
赣南医学院项目课题(No. YB201014)资助项目.
Progress in the Asymmetric Metal-Catalyzed 1,2-Addition Reactions of Arylboronic Acids with Ketones
Received date: 2013-05-27
Revised date: 2013-06-24
Online published: 2013-07-03
Supported by
Project supported by the Science Foundation of Gannan Medical University (No. YB201014).
罗人仕 , 廖建华 , 张剑 . 金属催化芳基硼酸与酮不对称1,2-加成反应研究进展[J]. 有机化学, 2013 , 33(11) : 2298 -2309 . DOI: 10.6023/cjoc201305044
A number of biologically active compounds and drugs contain the structural unit of chiral aryl-substituted tertiary alcohol. The catalytic asymmetric arylation of ketones is a powerful tool for the efficient construction of such compounds. Arylboron reagents have attracted considerable attention due to their availability, relative insensitivity to air and moisture, high functional group tolerance and generally low toxicity. Recent progress of the catalytic asymmetric arylation of highly activated ketones such as isatins, trifluoromethyl ketones, α-ketoesters, 1, 2-diketones and unactivated ketones is reviewed.
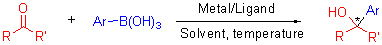
Key words: metal-catalyzed; arylboronic acid; ketone; asymmetric; 1,2-addition; progress
[1] For reviews,see: (a) Jacobsen,E.N.; Pfaltz,A.; Yamamoto,H.Comprehensive Asymmetric Catalysis,1 st ed.,Springer,Berlin,1999.
(b) Pu,L.; Yu,H.-B.Chem.Rev.2001,101,757.
(c) Fagnou,K.; Lautens,M.Chem.Rev.2003,103,169.
(d) Denmark,S.E.; Fu,J.Chem.Rev.2003,103,2763.
(e) Garcia,C.; Martin,V.S.Curr.Org.Chem.2006,10,1849.
(f) Riant,O.; Hannedouche,J.Org.Biomol.Chem.2007,5,873.
(g) Tian,P.; Dong,H.-Q.; Lin,G.-Q.ACS Catal.2011,95.
[2] (a) Romero,D.L.Ann.Rep.Med.Chem.1994,29,123.
(b) de Clercq,E.Rev.Med.Virol.2009,19,287.
(c) Wan,Z.-K.; Chenail,E.; Li,H.-Q.; Kendall,C.; Wang,Y.; Gingras,S.; Xiang,J.; Massefski,W.W.; Mansour,T.S.; Saiah,E.J.Org.Chem.2011,76,7048.
(d) Julian,L.; Wang,D.Z.; Bostick,T.; Caille,S.; Choi,R.; DeGraffenreid,M.; Di,Y.; He,X.; Hungate,R.W.; Jaen,J.C.; Liu,J.; Monshouwer,M.; McMinn,D.; Rew,Y.; Sudom,A.; Sun,D.; Tu,H.; Ursu,S.; Walker,N.; Yan,X.; Ye,Q.; Powers,J.P.J.Med.Chem.2008,51,3953.
(e) Kopecky,D.J.; Jiao,X.Y.; Fisher,B.; McKendry,S.; Labelle,M.; Piper,D.E.; Coward,P.; Shiau,A.K.; Escaron,P.; Danao,J.; Chai,A.; Jaen,J.; Kayser,F.Bioorg.Med.Chem.Lett.2012,22,2407.
[3] (a) Marshall,R.L.; Muderawan,I.W.; Young,D.J.J.Chem.Soc.,Perkin Trans.2 2000,957.
(b) Li,C.-J.; Meng,Y.J.Am.Chem.Soc.2000,122,9538.
[4] (a) Dosa,P.I.; Ruble,J.C.; Fu,G.C.J.Org.Chem.1997,62,444.
(b) Huang,W.-S.; Hu,Q.-S.; Pu,L.J.Org.Chem.1999,64,7940.
(c) Bolm,C.; Hermanns,N.; Hildebrand,J.P.; Muniz,K.Angew.Chem.,Int.Ed.2000,39,3465.
(d) Bolm,C.; Rudolph,J.J.Am.Chem.Soc.2002,124,14850.
(e) Montserrat,F.; Xavier,V.; Lluis,S.; Miquel,A.P.; Antoni,R.J.Org.Chem.2004,69,2532.
(f) Braga,A.L.; LÜdtke,D.S.; Vargas,F.; Paixâo,M.W.A.Chem.Commun.2005,19,2512.
[5] Tomita,D.; Wada,R.; Kanai,M.; Shibasaki,M.J.Am.Chem.Soc.2005,127,4138.
[6] Weber,B.; Seebach,D.Tetrahedron 1994,50,7473.
[7] Sakai,M.; Ueda,M.; Miyaura,N.Angew.Chem.,Int.Ed.1998,37,3279.
[8] Using rhodium catalysis: (a) Sakai,M.; Ueda,M.; Miyaura,N.Angew.Chem.,Int.Ed.1998,37,3279.
(b) Focken,T.; Rudolph,J.; Bolm,C.Synthesis 2005,429.
(c) Suzuki,K.; IshⅡ,S.; Kondo,K.; Aoyama,T.Synlett 2006,648.
(d) Suzuki,K.; Kondo,K.; Aoyama,T.Synthesis 2006,1360.
(e) Arao,T.; Suzuki,K.; Kondo,K.; Aoyama,T.Synthesis 2006,3809.
(f) Duan,H.F.; Xie,J.H.; Shi,W.J.; Zhang,Q.; Zhou,Q.L.Org.Lett.2006,8,1479.
(g) Jagt,R.B.C.; Toullec,P.Y.; de Vries,J.G.; Feringa,B.L.; Minnaard,A.J.Org.Biomol.Chem.2006,4,773.
(h) No€el,T.; Vandyck,K.; Van der Eycken,J.Tetrahedron 2007,63,12961.
(i) Nishimura,T.; Kumamoto,H.; Nagaosa,M.; Hayashi,T.Chem.Commun.2009,5713.
(j) Clarke,E.F.; Rafter,E.; M€uller-Bunz,H.; Higham,L.J.; Gilheany,D.G.J.Organomet.Chem.2011,696,3608.
[9] Using cobalt catalysis: Karthikeyan,J.; Jeganmohan,M.; Cheng,C.-H.Chem.Eur.J.2010,16,8989.
[10] Using nickel catalysis: (a) Arao,T.; Kondo,K.; Aoyama,T.Tetrahedron Lett.2007,48,4115.
(b) Yamamoto,K.; Tsurumi,K.; Sakurai,F.; Kondo,K.; Aoyama,T.Synthesis 2008,3585.
(c) Sakupai,F.; Kondo,K.; Aoyama,T.Chem.Pharm.Bull.2009,57,511.
(d) Sakurai,F.; Kondo,K.; Aoyama,T.Tetrahedron Lett.2009,50,6001.
[11] Using copper catalysis: Tomita,D.; Kanai,M.; Shibasaki,M.Chem.Asian J.2006,1,161.
[12] Using ruthenium catalysis: (a) Yamamoto,Y.; Kurihara,K.; Miyaura,N.Angew.Chem.,Int.Ed.2009,48,4414.
(b)Yamamoto,Y.; Shirai,T.; Watanabe,M.; Kurihara,K.; Miyaura,N.Molecules 2011,16,5020.
(c) Yamamoto,Y.; Shirai,T.; Miyaura,N.Chem.Commun.2012,48,2803.
(d) Li,K.; Hu,N.-F.; Luo,R.-S.; Yuan,W.-C.; Tang,W.J.Org.Chem.2013,78,6350.
[13] Zhang,R.; Xu,Q.; Zhang,X.; Zhang,T.; Shi,M.Tetrahedron: Asymmetry 2010,21,1928.
[14] Luo,F.; Pan,C.D.; Cheng,J.Chin.J.Org.Chem.2010,30,633 (in Chinese).(罗芳,潘长多,成江,有机化学,2010,30,633.)
[15] (a) Shintani,R.; Inoue,M.; Hayashi,T.Angew.Chem.,Int.Ed.2006,45,3353.
(b) Toullec,P.Y.; Jagt,R.B.C.; de Vries,J.G.; Feringa,B.L.; Minnaard,A.J.Org.Lett.2006,8,2715.
(c) Shintani,R.; Takatsu,K.; Hayashi,T.Chem.Commun.2010,46,6822.
(d) Martina,S.L.X.; Jagt,R.B.C.; de Vries,J.G.; Feringa,B.L.; Minnaard,A.J.Chem.Commun.2006,4093.
(e) Duan,H.F.; Xie,J.H.; Qiao,X.C.; Wang,L.X.; Zhou,Q.L.Angew.Chem.,Int.Ed.2008,47,4351.
(f) Cai,F.; Pu,X.; Qi,X.; Lynch,V.; Radha,A.; Ready,J.M.J.Am.Chem.Soc.2011,133,18066.
(g) Zhu,T.-S.; Jin,S.-S.; Xu,M.-H.Angew.Chem.,Int.Ed.2012,51,780.
(h) Feng,X.; Nie,Y.; Yang,J.; Du,H.Org.Lett.2012,14,624.
[16] Toullec,P.Y.; Jagt,R.B.C.; Vries,J.G.; Feringa,B.L.; Min-naard,A.J.Org.Lett.2006,8,2715.
[17] Jagt,R.B.C.; Toullec,P.Y.; de Vries,J.G.; Feringa,B.L.; Minnaard,A.J.Org.Biomol.Chem.2006,4,773.
[18] Shintani,R.; Inoue,M.; Hayashi,T.Angew.Chem.,Int.Ed.2006,45, 3353.
[19] Ackermann,L.Modern Arylation Methods,Wiley-VCH,Wein-heim,Germany,2009,pp.271~310.
[20] Lai,H.S.; Huang,Z.Y.; Wu,Q.; Qin,Y.J.Org.Chem.2009,74,283.
[21] Gui,J.Y.; Chen,G.H.; Cao,P.; Liao,J.Tetrahedron: Asymmetry 2012,23,554.
[22] (a) Yamamoto,Y.; Kurihara,K.; Miyaura,N.Angew.Chem.,Int.Ed.2009,48,4414.
(b) Yamamoto,Y.; Shirai,T.; Watanabe,M.; Kurihara,K.; Miyaura,N.Molecules 2011,16,5020.
(c) Yamamoto,Y.; Shirai,T.; Miyaura,N.Chem.Commun.2012,48,2803.
[23] Yamamoto,Y.; Yohd,M.; Shirai,T.; Ito,H.; Miyaura,N.Chem.Asian J.2012,7,2246.
[24] (a) Kaur,H.; Zinn,F.K.; Stevens,E.D.; Nolan,S.P.Organometallics 2004,23,1157.
(b) Diez-Gonzalez,S.; Kaur,H.; Zinn,F.K.; Stevens,E.D.; Nolan,S.P.J.Org.Chem.2005,70,4784.
(c) Lebel,H.; Davi,M.; Diez-Gonzalez,S.; Nolan,S.P.J.Org.Chem.2007,72,144.
(d) Laitar,D.S.; Tsui,E.Y.; Sadighi,J.P.J.Am.Chem.Soc.2006,128,11036.
(e) Jurkauskas,V.; Sadighi,J.P.; Buchwald,S.L.Org.Lett.2003,5,2417.
(f) Laitar,D.S.; MÜller,P.; Sadighi,J.P.J.Am.Chem.Soc.2005,127,17196.
[25] Shintani,R.; Takatsu,K.; Hayashi,T.Chem.Commun.2010,46,6822.
[26] Liu,Z.; Gu,P.; Shi,M.; McDowell,P.; Li,G.G.Org.Lett.2011,13,2314.
[27] Martina,S.L.X.; Jagt,R.B.C.; Vries,J.G.; Feringa B.L.; Minnaard,A.J.Chem.Commun.2006,4093.
[28] Jumde,V.R.; Facchetti,S.; Iuliano,A.Tetrahedron: Asymmetry 2010,21,2775.
[29] Luo,R.-S.; Li,K.; Hu,Y.L.; Tang,W.J.Adv.Synth.Catal.2013,355,1297.
[30] (a) Kiesewetter,D.O.; Silverton,J.V.; Eckelman,W.C.J.Med.Chem.1995,38,1711.
(b) Kiesewetter,D.O.; Carson,R.E.; Jagoda,E.M.; Endres,C.J.; Der,M.G.; Herscovitch,P.; Eckelman,W.C.Bioorg.Med.Chem.1997,5,1555.
(c) Skaddan,M.B.; Kilbourn,M.R.; Snyder,S.E.; Sherman,P.S.; Desmond,T.J.; Frey,K.A.J.Med.Chem.2000,43,4552.
(d) Selent,J.; Brandt,W.; Pamperin,D.Goeber,B.Bioorg.Med.Chem.2006,14,1729.
[31] (a) He,P.; Lu,Y.; Hu,Q.-S.Tetrahedron Lett.2007,48,5283.
(b) He,P.; Lu,Y.; Dong,C.-G.; Hu,Q.-S.Org.Lett.2007,9,343.
[32] Xie,J.-H.; Zhu,S.-F.; Zhou,Q.-L.Chem.Soc.Rev.2012,41,4126.
[33] Duan,H.-F.; Xie,J.-H.Qiao,X.-C.Wang,L.-X.; Zhou,Q.-L.Angew.Chem.,Int.Ed.2008,47,4351.
[34] Cai,F.; Pu,X.T.; Qi,X.B.; Lynch,V.; Radha,A.; Ready,J.M.J.Am.Chem.Soc.2011,133,18066.
[35] (a) Jin,S.-S.; Wang,H.; Xu,M.-H.Chem.Commun.2011,47,7230.
(b) Qi,W.-Y.; Zhu,T.-S.; Xu,M.-H.Org.Lett.2011,13,3410.
(c) Thaler,T.; Guo,L.-N.; Steib,A.K.; Raducan,M.; Karaghiosoff,K.; Mayer,P.; Knochel,P.Org.Lett.2011,13,3182.
(d) Feng,X.Wang,Y.; Wei,B.; Yang,J.; Du,H.Org.Lett.2011,13,3300.
(e) Chen,G.; Gui,J.; Li,L.; Liao,J.Angew.Chem.,Int.Ed.2011,50,7681.
(f) Feng,X.; Wei,B.; Yang,J.; Du,H.Org.Biomol.Chem.2011,9,5927.
(g) Xue,F.; Li,X.; Wan,B.J.Org.Chem.2011,76,7256.
(h) Wang,Y.; Feng,X.; Du,H.Org.Lett.2011,13,4954.
[36] Zhu,T.-S.; Jin,S.-S.; Xu,M.-H.Angew.Chem.,Int.Ed.2012,51,780.
[37] Jung,J.-K.; Johnson,B.R.; Duong,T.; Decaire,M.; Uy,J.; Gharbaoui,T.; Boatman,P.D.; Sage,C.R.; Chen,R.; Richman,J.G.; Connolly,D.T.; Semple,G.J.Med.Chem.2007,50,1445.
[38] Singh,S.B.; Zink,D.L.; Quamina,D.S.; Pelaez,F.; Teran,A.; Felock,P.; Hazuda,D.J.Tetrahedron Lett.2002,43,2351.
[39] Ganci,G.R.; Chisholm,J.D.Tetrahedron Lett.2007,48,8266.
[40] Feng,X.Q.; Nie,Y.Z.; Yang,J.; Du,H.F.Org.Lett.2012,14,624.
[41] Liu,G.X.; Lu,X.Y.J.Am.Chem.Soc.2006,128,16504.
[42] Gallego,G.M.; Sarpong,R.Chem.Sci.2012,3,1338.
[43] Low,D.W.; Pattison,G.; Wieczysty,M.D.; Churchill,G.H.; Lam,H.W.Org.Lett.2012,14,2548.
[44] Bouffard,J.; Itami,K.Org.Lett.2009,11,4410.
[45] Korenaga,T.; Ko,A.; Uotani,K.; Tanaka,Y.; Sakai,T.Angew.Chem.,Int.Ed.2011,50,10703.
[46] Liao,Y.-X.; Xing,C.-H.; Hu,Q.-S.Org.Lett.2012,14,1544.
/
| 〈 |
|
〉 |