

宾川乌头根中两个新的二萜生物碱
收稿日期: 2013-07-17
修回日期: 2013-08-13
网络出版日期: 2013-08-29
基金资助
国家自然科学基金(No. 21002084)、云南省自然科学基金(No. 2010CD017)和2012年云南大学省级大学生创新创业训练计划资助项目.
Two New Diterpenoid Alkaloids from the Roots of Aconitum duclouxii Levl
Received date: 2013-07-17
Revised date: 2013-08-13
Online published: 2013-08-29
Supported by
Project supported by the National Natural Science Foundation of China (No. 21002084), the Natural Science Foundation of Yunnan Province (No. 2010CD017), and an Undergraduates Innovative Experiment Project from Yunnan Province.
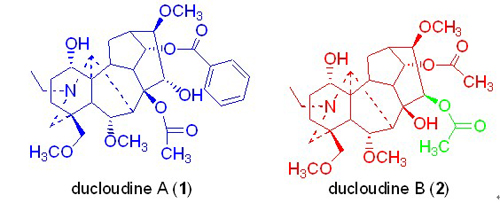
从宾川乌头(Aconitum duclouxii Levl.)的块根中分离得到了4个二萜生物碱,采用现代波谱技术,包括高分辨质谱、单晶X衍射、一维和二维核磁共振,将上述化合物分别鉴定为:ducloudine A(宾乌定甲) (1),ducloudine B(宾乌定乙) (2),14-O-乙酰尼奥宁(3)和乌头碱(4). 其中化合物1和2为新化合物,化合物3为首次从该植物中分离得到. 化合物1和2的抗肿瘤细胞活性实验表明,其对5种肿瘤细胞(HL-60,A549,SMMC-7721,MCF-7,SW480)均没有体外生长抑制活性(IC50 > 40 μmol/L).
关键词: 宾川乌头; 二萜生物碱; ducloudine A; ducloudine B; 细胞毒活性
尹田鹏 , 蔡乐 , 雷刚 , 董建伟 , 刘彦雄 , 丁中涛 . 宾川乌头根中两个新的二萜生物碱[J]. 有机化学, 2013 , 33(12) : 2528 -2532 . DOI: 10.6023/cjoc201307025
Two new C-19-diterpenoid alkaloids, ducloudines A (1) and B (2), together with two known alkaloids of 14-acetylneoline (3) and aconitine (4), were isolated from the roots of Aconitum duclouxii Levl. The structures of new alkaloids were established on the basis of spectral data (HR-MS, X-ray crystallographic analysis, 1D-and 2D-NMR). Cytotoxicities of compounds 1 and 2 were tested against five cancer cell lines (HL-60, A549, SMMC-7721, MCF-7, SW480). Results showed that compounds 1 and 2 had no cytotoxic effects on these five tumor cells (IC50 > 40 μmol/L).

Key words: Aconitum duclouxii; diterpenoid alkaloids; ducloudine A; ducloudine B; cytotoxicity
[1] Xiao, P. G.; Wang, F. P.; Gao, F.; Yan, L. P.; Chen, D. L.; Liu, Y. Acta Phytotaxom. Sin. 2006, 44, 1 (in Chinese). (肖培根, 王锋鹏, 高峰, 闫路平, 陈东林, 刘勇, 植物分类学报, 2006, 44, 1.)
[2] People’s Government of Dali Bai Autonomous Prefecture The Natural Resources of Chinese Medicinal Materials of Dali, Yunnan Ethnic Publishing House, Kunming, 1991, p. 77 (in Chinese). (大理白族自治州人民政府, 大理植物资源志, 云南民族出版社, 昆明, 1991, p. 77.)
[3] Wang, C. Y.; Chen, J. B.; Zhu, R. H. Acta Pharm. Sin. 1984, 19, 445 (in Chinese). (王崇云, 陈敬炳, 朱元龙, 朱任宏, 药学学报, 1984, 19, 445.)
[4] Cai, L.; Chen, D. L.; Liu, S. Y.; Wang, F. P. Chem. Pharm. Bull. 2006, 54, 779.
[5] Jiang, B. Y.; Lin, S.; Zhu, C. G.; Wang, S. J.; Wang, Y. N.; Chen, M. H.; Zhang, J. J.; Hu, J. F.; Chen, N. H.; Yang, Y. C.; Shi, J. G. J. Nat. Prod. 2012, 75, 1145.
[6] Tang, P.; Chen, D. L.; Jian, X. X.; Wang, F. P. Chin. Chem. Lett. 2007, 18, 704.
[7] Pelletier, S. W.; Mody, N. V.; Joshi, B. S.; Schramm, L. C. Alkaloids: Chemical and Biological Perspectives, Vol. 2, John Wiley & Sons, New York, 1984, p. 209.
[8] Peng, C. S.; Chen, D. L.; Chen, Q. H.; Wang, F. P. Chin. J. Org. Chem. 2005, 25, 1235 (in Chinese). (彭崇胜, 陈东林, 陈巧鸿, 王锋鹏, 有机化学, 2005, 25, 1235.)
[9] Ding, L. S.; Chen, Y. Z.; Wu, F. E. Planta Med. 1991, 57, 275.
[10] Takayama, H.; Hasegawa, S.; Sakai, S.; Hajiniwa, J.; Okamoto, T. Chem. Pharm. Bull. 1981, 29, 3078.
[11] Shim, S. H.; Kim, J. S.; Kang, S. S. Chem. Pharm. Bull. 2003, 51, 999.
[12] Gao, F.; Li, Y. Y.; Wang, D.; Huang, X.; Liu, Q. Molecules 2012, 17, 5187.
/
| 〈 |
|
〉 |