

三组分合成噁唑并[3,4-b]喹啉衍生物
收稿日期: 2013-09-08
修回日期: 2013-10-14
网络出版日期: 2013-10-28
基金资助
国家自然科学青年基金(No. 21102124)和江苏省“青蓝”工程(No. 12QLG006)资助项目.
Three-Component Synthesis of Oxazolo[3,4-b]quinoline Derivatives
Received date: 2013-09-08
Revised date: 2013-10-14
Online published: 2013-10-28
Supported by
Project supported by the National Natural Science Foundation of China (No. 21102124) and the Qing Lan Project of Jiangsu Province (No. 12QLG006).
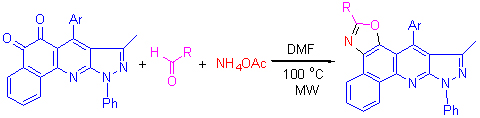
1,2-吖啶二酮衍生物、醛和醋酸铵在N,N-二甲基甲酰胺溶剂中经微波辐射,一步区域选择性地合成了噁唑并[3,4-b]喹啉衍生物. 该方法具有反应时间短(15~18 min),选择性高,操作简单和环境友好等优点. 产物的结构经红外光谱、核磁共振谱、质谱以及化合物4a的晶体经X射线衍射分析予以确证.
关键词: 微波辐射; 噁唑并[3,4-b]喹啉衍生物; 五环稠杂环; 三组分反应
佟光进 , 范威 , 姜波 . 三组分合成噁唑并[3,4-b]喹啉衍生物[J]. 有机化学, 2013 , 33(12) : 2578 -2582 . DOI: 10.6023/cjoc201309014
A series of oxazolo[3,4-b]quinoline derivatives were regioselectively synthesized by the three component reaction of acridin-1,2-dione derivatives with aldehydes and ammonium acetate in N,N-dimethylformamide (DMF) under microwave irradiation. This method has the advantages of short reaction times (15~18 min), excellent selectivity, and simple operation as well as environmental friendly. The structures of the products were identified by IR, NMR, HRMS, and the crystal of compound 4a was confirmed by X-ray diffraction.

[1] Alagarsamy, V.; Pathak, U. S. Bioorg. Med. Chem. 2007, 15, 3457.
[2] Murugan, V.; Kulkarni, M.; Anand, R. M.; Kumar, E. P.; Suresh, B.; Reddy, V. M. Asian J. Chem. 2006, 18, 900.
[3] Alagarsamy, V.; Raja S., V.; Dhanabal, K. Bioorg. Med. Chem. 2007, 15, 235.
[4] Selvam, P.; Girija, K.; Nagarajan, G.; De Clerco, E. Indian J. Pharm. Sci. 2005, 67, 484.
[5] Godfrey, A. A. A. WO 2005012260, 2005 [Chem. Abstr. 2005, 142, 198095].
[6] Chaturvedula, P. V.; Chen, L.; Civiello, R.; Degnan, A. P.; Dubowchik, G. M.; Han, X.; Jiang, X. J.; Macor, J. E.; Poindexter, G. S.; Tora, G. O.; Luo, G. US 2007149503, 2007 [Chem. Abstr. 2007, 147, 118256].
[7] Alanine, A.; Gobbi, L. C.; Kolczewski, S.; Luebbers, T.; Peters, J. U.; Steward, L. US 2006293350, 2006 [Chem. Abstr. 2006, 146, 100721].
[8] Letourneau, J.; Riviello, C.; Ho, K. K.; Chan, J. H.; Ohlmeyer, M.; Jokiel, P.; Neagu, I.; Morphy, J. R.; Napier, S. E. WO 2006095014, 2006 [Chem. Abstr. 2006, 145, 315012].
[9] (a) Dalisay, D. S.; Rogers, E. W.; Edison, A. S.; Molinski, T. F. J. Nat. Prod. 2009, 72, 732.
(b) Hagelueken, G.; Albrecht, S. C.; Steinmetz, H.; Jansen, R.; Heinz, D. W.; Kalesse, M.; Schubert, W. D. Angew. Chem., Int. Ed. 2009, 48, 595.
(c) Jin, Z. Nat. Prod. Rep. 2006, 23, 464.
[10] (a) Vedejs, E.; Barda, D. A. Org. Lett. 2000, 2, 1033.
(b) Wan, C.; Zhang, J.; Wang, S.; Fan, J.; Wang, Z. Org. Lett. 2010, 12, 2338.
(c) Jiang, H.; Huang, H.; Cao, H.; Qi, C. Org. Lett. 2010, 12, 5561.
[11] (a) Ferrini, P. G.; Marxer, A. Angew. Chem., Int. Ed. 1963, 2, 99.
(b) Pan, Y.-M.; Zheng, F.-J.; Lin, H.-X.; Zhan, Z.-P. J. Org. Chem. 2009, 74, 3148.
(c) Young, G. L.; Smith, S. A.; Taylor, R. J. K. Tetrahedron Lett. 2004, 45, 3797.
[12] (a) Williams, D. R.; Lowder, D. P.; Gu, G.-Y.; Brooks, D. A. Tetrahedron Lett. 1997, 38, 331.
(b) Meyers, A. I.; Tavares, F. X. J. Org. Chem. 1996, 61, 8207.
(c) Uto, A. J. Y.; Wipf, P.; Reno, M. J.; Williams, D. R. Org. Lett. 2000, 2, 1165.
[13] (a) Derridj, F.; Djebbar, S.; Benali-Baitich, O.; Doucet, H. J. Organomet. Chem. 2008, 693, 135.
(b) Flegeau, E. F.; Popkin, M. E.; Greaney, M. F. Org. Lett. 2006, 8, 2495.
(c) Lee, K.; Counceller, C. M.; Stambuli, J. P. Org. Lett. 2009, 11, 1457.
[14] (a) Tietze, L. F.; Brasche, G.; Gerike, K. Domino Reactions in Organic Chemistry, Wiley-VCH, Weinheim, 2006.
(b) Aaron M. D.; Eric, F. Acc. Chem. Res. 2010, 43, 440.
(c) Li, J.; Wang, N.; Li, C. J.; Jia, X. S. Chem. Eur. J. 2012, 18, 9645.
(d) Li, J.; Wang, N.; Li, C. J.; Jia, X. S. Org. Lett. 2012, 14, 4994.
[15] (a) Fan, W.; Ye, Q.; Xu, H.-W.; Jiang, B.; Wang, S.-L.; Tu, S.-J. Org. Lett. 2013, 15, 2258.
(b) Jiang, B.; Li, Q.-Y.; Zhang, H.; Tu, S.-J.; Pindi, S.; Li, G. Org. Lett. 2012, 14, 700.
(c)Jiang, B.; Li, Y.; Tu, M.-S.; Wang, S.-L.; Tu, S.-J.; Li, G. J. Org. Chem. 2012, 77, 7497.
[16] (a) Jiang, B.; Zhang, G.; Ma, N.; Shi, F.; Tu, S.-J.; Kaur, P.; Li, G. Org. Biomol. Chem. 2011, 9, 3834.
(b) Wang, S.-L.; Zhang, G.; Jie, D.; Jiang, B.; Wang, X.-H.; Tu, S.-J. Comb. Chem. High Throughput Screening 2012, 15, 400.
[17] (a) Rajesh, S. M.; Bala, B. D.; Perumal, S.; Menendez J. C. Green Chem. 2011, 13, 3248.
(b) Wang, S.-L.; Wu, F.-Y.; Cheng, C.; Zhang, G.; Liu, Y.-P.; Jiang, B.; Shi, F.; Tu, S.-J. ACS Comb. Sci. 2011, 13, 135.
[18] (a) Mahdavinia, G. H.; Amani, A. M.; Sepehrian, H. Chin. J. Chem. 2012, 30, 703.
(b) Wang, L.; Yao, Y.; Wang, Y.; Zou, G. Chin. J. Chem. 2009, 27, 343.
/
| 〈 |
|
〉 |