

二醋酸碘苯促进的芳酮类化合物羰基邻位乙酰化反应研究
收稿日期: 2013-11-13
修回日期: 2013-12-14
网络出版日期: 2014-01-02
基金资助
广东省自然科学基金(No. S2012040007868)资助项目
α-Acetoxylation of Aromatic Ketones Mediated by Iodobenzene Diacetate
Received date: 2013-11-13
Revised date: 2013-12-14
Online published: 2014-01-02
Supported by
Project supported by the Natural Science Foundation of Guangdong Provine (No. S2012040007868).
以苯乙酮类化合物为原料,氧化锌(ZnO)为催化剂,二醋酸碘苯为氧化剂,通过对羰基邻位饱和sp3-C—H键的氧化与乙酰化,成功的构建出了α-乙酸基苯乙酮类衍生物,其结构经1H NMR和13C NMR得到了确认,并提出了可能的反应机理.
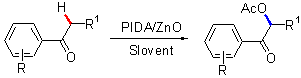
关键词: 二醋酸碘苯; 乙酰苯酮; 饱和sp3-C—H键氧化; 乙酰化
谭丽泉 , 刘卫兵 , 黄敏 , 余梅 . 二醋酸碘苯促进的芳酮类化合物羰基邻位乙酰化反应研究[J]. 有机化学, 2014 , 34(4) : 817 -820 . DOI: 10.6023/cjoc201311024
This paper reports a simple method for the synthesis of α-acetoxyacetophenone derivatives from acetophenones using ZnO as a catalyst and iodobenzene diacetate as an oxidant. This transformation undergoes two processes: the oxidation of saturated sp3-C—H bond and the acetoxylation of C=C bond. The structures of products were confirmed by IR, 1H NMR and 13C NMR. In addition, a possible reaction mechanism was proposed.
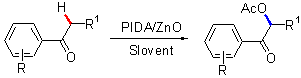
/
| 〈 |
|
〉 |