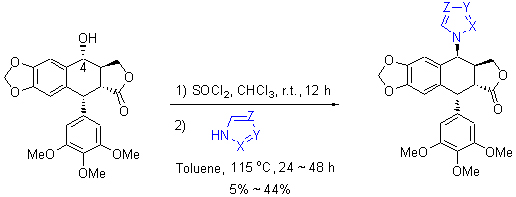
从鬼臼毒素出发,通过简洁的合成路线合成了9个新型的鬼臼毒素五元氮杂环类衍生物,其结构经1H NMR,13C NMR,IR,HR-ESI-MS以及X单晶衍射确定. 对合成的新化合物进行了体外抗肿瘤活性的筛选,结果表明,化合物10具有较好的细胞毒活性,IC50值为2.63~4.73 μmol/L,对4种细胞株的活性均优于顺铂,可以作为先导化合物作进一步的结构修饰和更深入的活性研究.
朱培芳
,
赵静峰
,
羊晓东
,
张洪彬
. 鬼臼毒素五元氮杂环类衍生物的合成及细胞毒活性研究[J]. 有机化学, 2014
, 34(6)
: 1167
-1171
.
DOI: 10.6023/cjoc201312029
Nine novel podophyllotoxin five-membered N-heterocyclic derivatives have been prepared from commercially podophyllotoxin. Their structures were confirmed by 1H NMR, 13C NMR, IR, HR-ESI-MS and X-ray crystallographic analysis. These derivatives have been evaluated in vitro against a panel of human tumor cell lines. Compound 10 (IC50: 2.63~4.73 μmol/L) showed superior cytotoxic activity compared with DDP (a clinically available anticancer drug) in four human tumor cell lines investigation, which is to be a lead compound for further structural modifications and activity research.
[1] Lv, M.; Xu, H. Mini-Rev. Med. Chem. 2011, 11, 901.
[2] He, F.; Liu, Z. C.; Zheng, K. C.; Yun, F. G. Chin. J. Org. Chem. 2001, 21, 278 (in Chinese).
(何峰, 刘宗潮, 郑康成, 云逢存, 有机化学, 2001, 21, 278.)
[3] Nagar, N.; Jat, R. K.; Saharan, R.; Verma, S.; Sharma, D.; Bansal, K. Pharmacophore 2011, 2(2), 124.
[4] Yousefzadi, M.; Sharifi, M.; Behmanesh, M.; Moyano, E.; Bonfill, M.; Cusido, R. M.; Palazon, J. Eng. Life Sci. 2010, 10(4), 281.
[5] Xu, H.; Lv, M.; Tian, X. Curr. Med. Chem. 2009, 16, 327.
[6] Zhang, Z.-J.; Tian, J.; Wang, L.-T.; Wang, M.-J.; Nan, X.; Yang, L.; Liu, Y.-Q.; Morris-Natschke, S. L.; Lee, K.-H. Bioorg. Med. Chem. Lett. 2014, 22(1), 204.
[7] Che, Z. P.; Yu, X.; Zhi, X. Y.; Fan, L. L.; Yao, X. J.; Xu, H. J. Agric. Food. Chem. 2013, 61(34), 8148.
[8] Shi, J. F.; Wang, Y. G. Chin. J. Org. Chem. 2003, 23, 1244 (in Chinese).
(施建峰, 王彦广, 有机化学, 2003, 23, 1244.)
[9] Xu, H.; Zhang, L.; Tian, X. Chin. J. Org. Chem. 2008, 28, 1243 (in Chinese).
(徐晖, 张雷, 田瑄, 有机化学, 2008, 28, 1243.)
[10] Umesha, B.; Basavaraju, Y. B. Eur. J. Org. Chem. 2013, 4(3), 235.
[11] Zeng, X. H.; Yang, X. D.; Zhang, Y. L.; Qing, C.; Zhang, H. B. Bioorg. Med. Chem. Lett. 2010, 20, 1844.
[12] CCDC 977808 contains the supplementary crystallographic data for compound 9. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/ data_request/cif.
[13] CCDC 977807 contains the supplementary crystallographic data for compound 10. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/ data_request/cif.