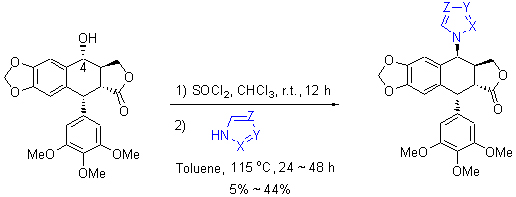
| [1] Lv, M.; Xu, H. Mini-Rev. Med. Chem. 2011, 11, 901.[2] He, F.; Liu, Z. C.; Zheng, K. C.; Yun, F. G. Chin. J. Org. Chem. 2001, 21, 278 (in Chinese).(何峰, 刘宗潮, 郑康成, 云逢存, 有机化学, 2001, 21, 278.)[3] Nagar, N.; Jat, R. K.; Saharan, R.; Verma, S.; Sharma, D.; Bansal, K. Pharmacophore 2011, 2(2), 124.[4] Yousefzadi, M.; Sharifi, M.; Behmanesh, M.; Moyano, E.; Bonfill, M.; Cusido, R. M.; Palazon, J. Eng. Life Sci. 2010, 10(4), 281.[5] Xu, H.; Lv, M.; Tian, X. Curr. Med. Chem. 2009, 16, 327.[6] Zhang, Z.-J.; Tian, J.; Wang, L.-T.; Wang, M.-J.; Nan, X.; Yang, L.; Liu, Y.-Q.; Morris-Natschke, S. L.; Lee, K.-H. Bioorg. Med. Chem. Lett. 2014, 22(1), 204.[7] Che, Z. P.; Yu, X.; Zhi, X. Y.; Fan, L. L.; Yao, X. J.; Xu, H. J. Agric. Food. Chem. 2013, 61(34), 8148.[8] Shi, J. F.; Wang, Y. G. Chin. J. Org. Chem. 2003, 23, 1244 (in Chinese).(施建峰, 王彦广, 有机化学, 2003, 23, 1244.)[9] Xu, H.; Zhang, L.; Tian, X. Chin. J. Org. Chem. 2008, 28, 1243 (in Chinese).(徐晖, 张雷, 田瑄, 有机化学, 2008, 28, 1243.)[10] Umesha, B.; Basavaraju, Y. B. Eur. J. Org. Chem. 2013, 4(3), 235.[11] Zeng, X. H.; Yang, X. D.; Zhang, Y. L.; Qing, C.; Zhang, H. B. Bioorg. Med. Chem. Lett. 2010, 20, 1844.[12] CCDC 977808 contains the supplementary crystallographic data for compound 9. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/ data_request/cif.[13] CCDC 977807 contains the supplementary crystallographic data for compound 10. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/ data_request/cif. |