

无催化剂条件下有效的四组分反应合成2,3-二氢噻吩衍生物
收稿日期: 2014-04-03
修回日期: 2014-04-29
网络出版日期: 2014-05-07
基金资助
国家自然科学基础研究基金(No. 21172188)和江苏省功能性材料绿色合成重点实验室开放课题(No. K201312)资助项目.
An Efficient Synthesis of 2,3-Dihydrothiophene Derivatives from Four-Component Reactions under Catalyst-Free Conditions
Received date: 2014-04-03
Revised date: 2014-04-29
Online published: 2014-05-07
Supported by
Project supported by the National Natural Science Foundation of China (No. 21172188), and the Open Foundation of Jiangsu Key Laboratory of Green Synthetic Chemistry for Functional Materials (No. K201312).
张金鹏 , 高亚年 , 陈明 , 姜灵 , 夏盛 , 荣良策 . 无催化剂条件下有效的四组分反应合成2,3-二氢噻吩衍生物[J]. 有机化学, 2014 , 34(9) : 1895 -1899 . DOI: 10.6023/cjoc201404009
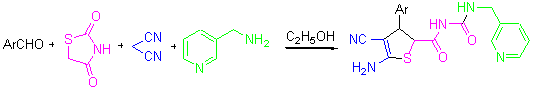
An efficient synthesis of 2,3-dihydrothiophene derivatives by the four-component reactions of aromatic aldehydes, thiazolidine-2,4-dione, malononitrile and pyridin-3-ylmethanamine has been reported. This reaction could be carried out smoothly without using any other catalysts in ethanol (95%) and the aromatic heterocyclic amine (pyridin-3-ylmethanamine) was first used for the synthesis of 2,3-dihydrothiophene derivatives. The advantages of this procure were mild reaction conditions, easy operation, and high yields. The products were identified by IR, 1H NMR and HRMS.
[1] Sperry Jeffrey, B.; Wright, D.L.Curr.Opin.Drug Discovery Dev.2005, 8, 723.
[2] Wu, C.; Decker, E.R.; Blok, N.; Bui, H.; You, T.J.; Wang, J.; Bourgoyne, A.R.; Knowles, V.; Berens, K.L.; Holland, G.W.; Brock, T.A.; Dixon, R.A.F.J.Med.Chem.2004, 47, 1969.
[3] Gewald, K.Angew.Chem.1961, 73, 114.
[4] Chakrabarti, J.K.; Hotten, T.M.; Tupper, D.E.Preparation of 2-Methylthienobenzodiazepine as Central Nervous System Agent (Lilly Industries Ltd., UK), Application, US, 11 pp, Cont-in-part of US Ser No 44, 844, abandoned.1997.
[5] Sun, J.; Zhang, L.L.; Xia, E.Y.; Yan, C.G.J.Org.Chem.2009, 74, 3398.
[6] Lu, G.P.; Zeng, L.Y.; Cai, C.Green Chem.2011, 13, 998.
[7] Tong, D.; Xia, S.; Tao, S.M.; Gao, L.J.; Feng, Y.J.; Rong, L.C.Res.Chem.Intermed.2013, 39, 561.
[8] Sun, J.; Xia, E.Y.; Yao, R.; Yan, C.G.Mol.Diversity 2011, 15, 115.
[9] Sun, J.; Wu, Q.; Xia, E.Y.; Yan, C.G.Synthesis 2010, 3987.
[10] Gao, L.J.; Xia, S.; Wu, N.; Tao, S.M.; Feng, Y.J.; Rong, L.C.Synth.Commun.2013, 43, 2590.
[11] Shi, D.Q.; Zou, Y.; Hu, Y.; Wu, H.J.Heterocycl.Chem.2011, 48, 896.
/
| 〈 |
|
〉 |