

一类新型大环内酯衍生物的合成及其生物活性研究
收稿日期: 2014-05-06
修回日期: 2014-07-28
网络出版日期: 2014-08-11
基金资助
北京理工大学基础研究基金(No.20131042006)资助项目.
Study of Synthesis and Biological Activities of a Novel Macrolide Derivatives
Received date: 2014-05-06
Revised date: 2014-07-28
Online published: 2014-08-11
Supported by
Project supported by the Basic Research Fund of Beijing Institute of Technology (No. 20131042006).
史大昕 , 冯雪 , 庄晓磊 , 柴洪新 , 刘霆 , 张奇 , 李加荣 . 一类新型大环内酯衍生物的合成及其生物活性研究[J]. 有机化学, 2014 , 34(12) : 2543 -2550 . DOI: 10.6023/cjoc201405007
Modification of molecular structure of pesticides is a common method to seek new efficient broad-spectrum insecticides. This study focused on modifying the structure of deglycosylated or partially deglycosylated spinosad. Eight new macrolide derivatives had been synthesized by acylation. Their structures were characterized by 1H NMR, 13C NMR and MS techniques. Their fungicidal and insecticidal activities were also evaluated. The results showed that all compounds synthesized had excellent insecticidal activities against lepidoptera, coleoptera, diptera, hemiptera and nematoda. Compound 3e at the concentration of 100 mg/L presented 100% insecticidal activity against hemiptera (e.g. Myzus persicae) and compounds 3f and 3h loading 200 μg presented certain inhibition against pseudomonas.
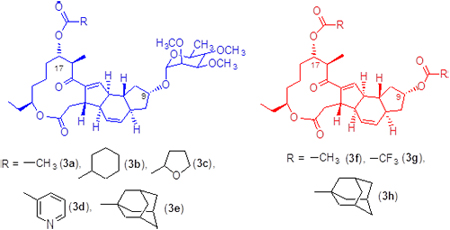
Key words: macrolide; acylate; insecticidal activity; fungicidal activity
[1] Yao, X. Y; Zhang, Y. X. Med. Recapitulate 2011, 32(7), 319 (in Chinese). (姚晓英, 张永信, 医药综述, 2011, 32(7), 319.)
[2] Weissman, K. J. Philos. Trans. R. Soc., A 2004, 362(1825), 2671.
[3] Floss, H. G. J. Ind. Microbiol. Biotechnol. 2001, 27(3), 183.
[4] Putnam, S. D.; Sader, H. S.; Farrell, D. J.; Biedenbach, D. J. Int. J. Antimicrob. Agents 2011, 37(1), 39.
[5] Jiang, L. J.; Or, Y. S. Antimicrob. Agents. Chemother. 2009, 53(8), 3218.
[6] Ge, H.; Shen, S. Y. World Clinical Drugs 2007, 28(6), 376 (in Chinese). (葛涵, 沈舜义, 世界临床药物, 2007, 28(6), 376.)
[7] Lee, H. Y.; Chung, H. S.; Hang, C.; Khosla, C.; Walsh, C. T.; Kahne, D.; Walker, S. J. Am. Chem. Soc. 2004, 126(32), 9924.
[8] Rospide, M. F.; Biedenbach, D. J. Int. J. Antimicrob. Agents 2001, 17(6), 451.
[9] Garza, R. G.; Xiong, L.; Zong, P. J. Bacteriol. 2001, 183, 6898.
[10] Farrell, D. J.; Sader, H. S.; Castanheira, M. Int. J. Antimicrob. Agents 2010, 35(6), 537.
[11] Holt, K. M.; Opit, G. P.; Nechols, J. R. Exp. Appl. Acarol. 2006, 38, 141.
[12] Mota, S. D.; Hollingworth, R. M.; Grafius, E. J. Pest Manage. Sci. 2006, 62, 30.
[13] Du, S. T.; Zhu, M. J.; Lang, S. Z. Agrochemicals 2005, 44(10), 441 (in Chinese). (杜顺堂, 朱明军, 梁世中, 农药, 2005, 44(10), 441.)
[14] Thompson, G. D.; Dutton, R.; Sparks, T. C. Pest Manage. Sci. 2000, 56(8), 696.
[15] Ou, X. M.; Liu, S. Y.; Pei, H.; Ma, D. Y.; Li, M.; Liu, L. J.; Wang, X. Z.; Yi, Z. H.; Bai, J. J.; Yu, K. CN 102977166, 2013 [Chem. Abstr. 2013, 158, 495574].
[16] Li, R.; Wang, Q. M.; Huang, R. Q. Chin. J. Pestic. Sci. 2003, 5(2), 1 (in Chinese). (李如, 汪清民, 黄润秋, 农药学学报, 2003, 5(2), 1.)
[17] Thomas, C. Pestic. Biochem. Phys. 2000, 67(3), 187.
[18] Sparks, T. C.; Crouse, G. D.; Dripps, J. E.; Anzeveno, P.; Martynow, J.; Deamicis, V.; Gifford, J. J. Comput.-Aided Mol. Des. 2008, 22, 393.
[19] Waldron, C.; Matsushima, P.; Rosteck, P. R.; Broughton, M. C.; Turner, J.; Madduri, K; Crawdord, K. P.; Merlo, D. J.; Baltz, R. H. J. Chem. Biol. 2001, 8(5), 487.
[20] Deamicis, C. V.; Anzeveno, P. B.; Martynow, J. G.; McLaren, K. L.; Green, F. R., III; Sparks, T. C.; Kirst, H. A.; Creemer, L. C.; Worden, T. V.; Schoonover, J. R., Jr.; Gifford, J. M.; Hatton, C. J.; Hegde, V. B.; Crouse, G. D.; Thoreen, B. R.; Ricks, M. J. US 006001981, 1999 [Chem. Abstr. 1999, 132, 35986].
[21] Podhorez, D. E.; Roth, G. A.; Molzahn, D. C.; Adaway, T. US 2008/0108800, 2008 [Chem. Abstr. 2008, 148, 517925].
[22] Creemer, L. C.; Kirst, H. A.; Paschal, J. W. J. Antibiot. 1998, 51(8), 795.
[23] Wanka, L.; Iqbal, K.; Schreiner, P. R. Chem. Rev. 2013, 113(5), 3516.
[24] Wang, H.; Yang, Z. K.; Fan, Z. J.; Wu, Q. J.; Zhang, Y. J.; Mi, N.; Wang, S. X.; Zhang, Z. C. Agric. Food Chem. 2011, 59, 628.
/
| 〈 |
|
〉 |