

卤代芳烃的无溶剂微波法高效合成有机锡化合物及原位自身偶联反应
收稿日期: 2014-08-22
修回日期: 2014-10-09
网络出版日期: 2014-11-03
Highly Efficient Synthesis of Stannanes from Aryl halides and Their in-situ Homo-Coupling under Microwave-Irradiated and Solvent-Free Conditions
Received date: 2014-08-22
Revised date: 2014-10-09
Online published: 2014-11-03
潘春娇 , 刘敏 , 段新红 . 卤代芳烃的无溶剂微波法高效合成有机锡化合物及原位自身偶联反应[J]. 有机化学, 2015 , 35(2) : 472 -477 . DOI: 10.6023/cjoc201408026
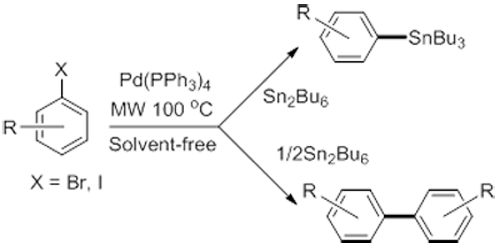
A novel approach for the synthesis of a variety of aryl stannanes from aryl halides and Sn2Bu6 by using Pd(PPh3)4 as catalyst is developed under microwave-irradiated and solvent-free conditions. This method offers the advantages of not only being simple, rapid and highly efficient, but also producing little byproduct and reducing pollution resulting from the use of solvent. Also, the procedure is applicable to the in-situ homo-coupling of aryl halides.
Key words: microwave heating; solvent-free; stannanes; homo-coupling; in-situ
[1] (a) Lu, A. L.; Wang, F. J.; Huang, D. F.; Wang, K. H.; Su, Y. P.; Xu, Y. L.; Hu, Y. L. Chin. J. Org. Chem. 2014, 34, 948 (in Chinese). (陆爱玲, 王凤娇, 黄丹凤, 王克虎, 苏瀛鹏, 徐艳丽, 胡雨来, 有机化学, 2014, 34, 948.)
(b) Zhang, X. Y.; Song, H. B.; Tang, L. F. Acta Chim. Sinica 2011, 69, 2567 (in Chinese). (张晓燕, 宋海滨, 唐良富, 化学学报, 2011, 69, 2567.)
(c) Zhang, Z. H.; Xu, W. J.; Lu, Y. B.; Xiong, Y. Q.; An, D. L.; Peng, Z. H. Acta Chim. Sinica 2007, 65, 2905 (in Chinese). (张正华, 徐伟箭, 卢彦兵, 熊远钦, 安德烈, 彭志鸿, 化学学报, 2007, 65, 2905.)
(d) Song, X. Q.; Zhong, G. Y.; Huang, Y. Q.; Xie, Q. L. Chin. J. Org. Chem. 2002, 22, 735 (in Chinese). (宋雪清, 钟桂云, 黄艳琴, 谢庆兰, 有机化学, 2002, 22, 735.)
[2] (a) Zeng, J.; Liu, K. M.; Duan, X. F. Org. Lett. 2013, 15, 5342.
(b) Cho, S. H. Chem. Soc. Rev. 2011, 40, 5068.
(c) Li, W. Y.; Zhao, D. M.; Xiong, X. Q.; Ma, Q. Q.; Cheng, M. S. Chin. J. Org. Chem. 2011, 31, 784 (in Chinese). (李文燕, 赵冬梅, 熊绪琼, 马倩倩, 程卯生, 有机化学, 2011, 31, 784.)
(d) Wang, D. P.; Zhang, X. D.; Liang, Y.; Li, J. H. Chin. J. Org. Chem. 2006, 26, 19 (in Chinese). (王德平, 张旭东, 梁云, 李金恒, 有机化学, 2006, 26, 19.)
(e) Nicolaou, K. C. Angew. Chem., Int. Ed. 2005, 44, 4442.
(f) Yu, Z. K.; Wang, S. H. Chin. J. Org. Chem. 1993, 13, 579 (in Chinese). (余正坤, 王世华, 有机化学, 1993, 13, 579.) (g) Wang, Z. Y.; Guo, X. B. Chin. J. Org. Chem. 1989, 9, 124 (in Chinese). (王昭煜, 郭秀斌, 有机化学, 1989, 9, 124.)
[3] (a) Ono, M. Chem. Pharm. Bull. 2009, 57, 1029.
(b) Qu, W. C. J. Med. Chem. 2007, 50, 3380.
(c) Zhuang, Z. P. J. Med. Chem. 2001, 44, 1905.
[4] (a) Hayashi, T.; Ishigedani, M. Tetrahedron 2001, 57, 2589.
(b) Knochel, P.; Singer, R. D. Chem. Rev. 1993, 93, 2117.
(c) Iddon, B.; Lim, B. L. J. Chem. Soc., Perkin Trans. 1 1983, 271.
(d) Gilman, H.; Rosenberg, S. D. J. Am. Chem. Soc. 1953, 75, 2507.
[5] (a) Handy, C. J.; Manoso, A. S.; McElroy, W. T.; Seganish, W. M.; DeShong, P. Tetrahedron 2005, 61, 12201.
(b) Zhu, X.; Blough, B. E.; Caroll, F. I. Tetrahedron Lett. 2000, 41, 9219.
(c) Sandosham, J.; Undheim, K. Acta Chem. Scand. 1989, 43, 684.
(d) Azizian, H.; Eaborn, C.; Pidcock, A. J. Organomet. Chem. 1981, 215, 49.
[6] (a) Duan, X. H.; Qiao, J. P.; Yang, Y.; Cui, M. C.; Zhou, J. N.; Liu, B. L. Bioorg. Med. Chem. 2010; 18: 1337.
(b) Suzuki, M.; Doi, H.; Kato, K.; Bjoèrkman, M.; Laêngstroèm, B.; Watanabe, Y.; Noyori, R. Tetrahedron 2000, 56, 8263.
[7] (a) Li, X. L.; Wang, W.; Li, R.; Zhang, P. Z.; Chen, H. Chin. J. Org. Chem. 2012, 32, 1519 (in Chinese). (李小六, 王玮, 李锐, 张平竹, 陈华, 有机化学, 2012, 32, 1519.)
(b) Kappe, C. O. Chem. Soc. Rev. 2008, 37, 1127.
(c) Dallinger, D. Chem. Rev. 2007, 107, 2563.
(d) Roberts, B. A. Acc. Chem. Res. 2005, 38, 653.
(e) Melucci, M.; Barbarella, G.; Sotgiu, G. J. Org. Chem. 2002, 67, 8877.
(f) Lu, M. W.; Hu, W. X. Chin. J. Org. Chem. 1995, 15, 561 (in Chinese). (陆模文, 胡文祥, 有机化学, 1995, 15, 561.)
[8] Tan, X.; Zhou, Z. J.; Zhang, J. X.; Duan, X. H. Eur. J. Org. Chem. 2014, 5153.
[9] (a) Shi, Z. C.; Zhao, Z. G.; Li, H.; Tan, J. Chin. J. Org. Chem. 2014, 34, 572 (in Chinese). (石治川, 赵志刚, 李晖, 谭炯, 有机化学, 2014, 34, 572.)
(b) Lai, Q. Y.; Liao, R. S.; Wu, S. Y.; Zhang, J. X.; Duan, X. H. New J. Chem. 2013, 37, 4069.
(c) Moseley, J. D.; Kappe, C. O. Green Chem. 2011, 13, 794.
(d) Lu, J.; Ge, H. G.; Bai, Y. J. Chin. J. Org. Chem. 2002, 22, 782 (in Chinese). (路军, 葛红光, 白银娟, 有机化学, 2002, 22, 782.)
(e) Varma, R. S. Green Chem. 1999, 1, 43.
[10] (a) Wang, B.; Qin, L.; Neumann, K. D.; Uppaluri, S.; Cerny, R. L.; DiMagno, S. G. Org. Lett. 2010, 12, 3352.
(b) Mase, N.; Nakai, Y.; Ohara, N.; Yoda, H.; Takabe, K.; Tanaka, F.; Barbas III, C. F. J. Am. Chem. Soc. 2006, 128, 734.
(c) Narayan, S.; Muldoon, J.; Finn, M. G.; Fokin, V. V.; Kolb, H. C.; Sharpless, K. B. Angew. Chem., Int. Ed. 2005, 44, 3275.
[11] (a) Rubio, S.; León, F.; Quintana, J.; Cutler, S.; Estévez, F. Eur. J. Med. Chem. 2012, 55, 284.
(b) Hayakawa, M.; Kaizawa, H.; Moritomo, H.; Koizumi, T.; Ohishi, T.; Okada, M.; Ohta, M.; Tsukamoto, S.; Parker, P.; Workman, P.; Waterfield, M. Bioorg. Med. Chem. 2006, 14, 6847.
(c) Shinji, C.; Nakamura, T.; Maeda, S.; Yoshida, M.; Hashimoto, Y.; Miyachi, H. Bioorg. Med. Chem. Lett. 2005, 15, 4427.
(d) Mathis, C. A.; Wang, Y. M.; Holt, D. P.; Huang, G. F.; Debnath, M. L.; Klunk, W. E. J. Med. Chem. 2003, 46, 2740.
[12] (a) Tsvelikhovsky, D.; Blum, J. Eur. J. Org. Chem. 2008, 2417.
(b) Li, J.-H.; Liang, Y.; Wang, D.-P.; Liu, W.-J.; Xie, Y.-X.; Yin, D.-L. J. Org. Chem. 2005, 70, 2832.
[13] Tang, P. P.; Furuya, T.; Ritter, T. J. Am. Chem. Soc. 2010, 132, 12150.
[14] Wei, J. F.; Jiao, J.; Feng, J. J.; Lv, J.; Zhang, X. R.; Shi, X. Y.; Chen, Z. G. J. Org. Chem. 2009, 74, 6283.
[15] Pinault, T.; Chérioux, F.; Therrien, B.; S?ss-Fink, G. Heteroat. Chem. 2004, 15, 121.
[16] The compound can also be synthesized by the other method, see: (a) Wang, M.; Zhang, T. T.; Song, Z. G. Chin. J. Org. Chem. 2010, 30, 740 (in Chinese). (王敏, 张婷婷, 宋志国, 有机化学, 2010, 30, 740.)
(b) Hayakawa, M.; Kaizawa, H.; Moritomo, H.; Koizumi, T.; Ohishi, T.; Okada, M.; Ohta, M.; Tsukamoto, S.; Parker, P.; Workman, P.; Waterfield, M. Bioorg. Med. Chem. 2006, 14, 6847.
[17] The compound can also be synthesized by the other method, see: (a) Ke, F.; Wu, W.; Lin, C.; Li, P. Chin. J. Org. Chem. 2013, 33, 2559 (in Chinese). (柯方, 吴雯, 林晨, 李鹏, 有机化学, 2013, 33, 2559.)
(b) Mathis, C. A.; Wang, Y. M.; Holt, D. P.; Huang, G. F.; Debnath, M. L.; Klunk, W. E. J. Med. Chem. 2003, 46, 2740.
[18] Lee, P. H.; Seomoon, D.; Lee, K. Org. Lett. 2005, 7, 343.
[19] (a) Qian, Y. L.; Wang, C.; Tao, X. C.; Huang, J. L. Chin. J. Org. Chem. 2003, 23, 1264 (in Chinese). (钱延龙, 王晨, 陶晓春, 黄吉玲, 有机化学, 2003, 23, 1264.)
(b) Parry, P. R.; Wang, C. S.; Batsanov, A. S.; Bryce, M. R.; Tarbit, B. J. Org. Chem. 2002, 67, 7541.
[20] Billingsley, K.; Buchwald, S. L. J. Am. Chem. Soc. 2007, 129, 3358.
[21] Rai, C.; Braunwarth, J. B. J. Org. Chem. 1961, 26, 3434.
[22] Cahiez, G.; Moyeux, A.; Buendia, J.; Duplais, C. J. Am. Chem. Soc. 2007, 129, 13788.
/
| 〈 |
|
〉 |