

含氟喹啉酰胺类化合物的合成及杀菌活性
收稿日期: 2015-04-18
修回日期: 2015-06-03
网络出版日期: 2015-06-12
基金资助
“十二五”国家科技支撑计划(No. 2011BAE06B02-04)资助项目.
Synthesis and Fungicidal Activity of a Series of Fluorinated Quinoline Amide Compounds
Received date: 2015-04-18
Revised date: 2015-06-03
Online published: 2015-06-12
Supported by
Project supported by the National Key Technologies R&D Program (No. 2011BAE06B02-04).
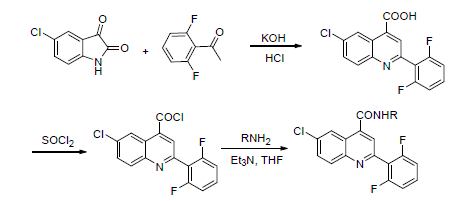
为寻求具有较高生物活性的农药先导化合物, 笔者采用活性亚结构拼接方法设计合成一类含氟喹啉酰胺类化合物. 以5-氯靛红与2,6-二氟苯乙酮为起始原料, 经过环合、酰化等多步反应, 得到13个未见文献报道的含氟喹啉酰胺类化合物. 其结构均经1H NMR和HRMS确证. 初步生物活性测定结果表明, 在50 mg/L质量浓度下, 目标化合物对小麦全蚀病(Gaeumannomyces graminis)、小麦赤霉病(Fusahum graminearum sehw)、小麦纹枯病(Rhizoctonia cerealis)、水稻稻瘟病(Pyricularia grisea)均具有杀菌活性. 而部分化合物对小麦全蚀病(Gaeumannomyces graminis)的杀菌活性达到90%以上.
倪芸 , 许天明 , 钟良坤 , 孔晓燕 , 史建俊 , 刘幸海 , 孔小林 , 姬文娟 , 谭成侠 . 含氟喹啉酰胺类化合物的合成及杀菌活性[J]. 有机化学, 2015 , 35(10) : 2218 -2222 . DOI: 10.6023/cjoc201504025
To find new fungicidal lead compounds with high bioactivity, novel lead compounds were designed by active group splicing. The synthetic routes are as follows: 6-chloro-2-(2,6-difluorophenyl)quinoline-4-carbonyl chloride (7) was synthesized using 5-chlorine isatin (4) and 2,6-difluoro acetophenone (5) as raw materials by cyclization, acyl chlorination and other step reactions. Then 13 novel title compounds were synthesized by acylation of 6-chloro-2-(2,6-difluorophenyl)-quino-line-4-carbonyl chloride (7) with substituted anilines (8). The structures of the compounds were confirmed by HRMS and 1H NMR. The preliminary fungicidal activity showed that all the compounds exhibit fungicidal activity against Gaeumannomyces graminis and Fusahum graminearum sehw, Rhizoctonia cerealis and Pyricularia grisea at concentration of 50 mg/L, part of compounds against Gaeumannomyces graminis were more than 90% at concentration of 50 mg/L.

Key words: fluorinated quinoline; amide compounds; synthesis; fungicidal activity
[1] Liu, X. H.; Xu, X. Y.; Tan, C. X.; Weng, J. Q.; Xin, J. H.; Chen, J. Pest Manage Sci. 2015, 71, 292.
[2] Liu, X. H.; Sun, Z. H.; Yang, M. Y.; Tan, C. X.; Weng, J. Q.; Zhang, Y. G.; Ma, Y. Chem. Biol. Drug Des. 2014, 84, 342.
[3] Nicholson, A. N.; Stone, B. M.; Clarke C. H. J. Clin. Pharmacol. 1977, 4, 567.
[4] Zayed, M. E. M.; El-Shishtawy, R. M.; Elroby, S. A.; Obaid, A. Y.; Al-amshany, Z. M. Int. J. Mol. Sci. 2015, 16, 3804.
[5] Wang, L.; ?witalska, M.; Wang, N.; Du, Z.-J.; Fukumoto, Y.; Diep, N. K.; Kiguchi, R.; Nokami, J.; Wietrzyk, J.; Inokuchi, T. Molecules 2014, 19, 19021.
[6] Fragoulis, G.; Merli, A.; Reeves, G.; Meregalli, G.; Stenberg, K.; Tanaka, T.; Capri, E. Pest Manage. Sci. 2011, 67, 656.
[7] Liu, C. L. World Pesticide: Fungicide, Chemical Industry Press, Beijing, 2006, p. 251 (in Chinese). (刘长令, 世界农药大全杀菌剂卷, 化学工业出版社, 北京, 2006, p. 251.)
[8] Nakano, H.; Masumizu, T. JP 2009091320, 2009 [Chem. Abstr. 2010, 150, 472698].
[9] Hackler, R. E.; Jourdan G. P.; Johnson, P. L.; Thoreen, B. R.; Samaritoni, J. G. WO 9304580, 1993 [Chem. Abstr. 1993, 119, 139111].
[10] (a) Liu, X. H.; Weng, J. Q.; Wang, B. L.; Li, Y. H.; Tan, C. X.; Li, Z. M. Chem. Res. Intermed. 2014, 40, 2605. (b) Yang, M. Y.; Zhao, W.; Sun, Z. H.; Tan, C. X.; Weng, J. Q.; Liu, X. H. Lett. Drug Des. Discovery 2015, 12, 314. (c) Xie, F.; Liu, T. T.; Yang, G.; Yuan, J.; Kong, X. L.; Xu, T. M.; Tan, C. X. Chin. J. Org. Chem. 2013, 33, 2596 (in Chinese). (谢峰, 刘婷婷, 杨果, 袁静, 孔小林, 许天明, 谭成侠, 有机化学, 2013, 33, 2596.)(d) Yan, S. L.; Yang, M. Y.; Sun, Z. H.; Min, L. J.; Tan, C. X.; Weng, J. Q.; Wu, H. K.; Liu, X. H. Lett. Drug Des. Discov. 2014, 11, 940. (e) Sun, G.-X.; Sun, Z.-H.; Yang, M.-Y.; Liu, X.-H.; Ma, Y.; Wei, Y.-Y. Molecules 2013, 18, 14876.
[11] Karen, L.; Daniel, D. S. Synthesis 1993, 993.
[12] Yu, M. Q.; Liu, G. Y.; Y, X. R.; Chen, Q. Y.; Di, N. Fine Chem. Intermed. 2011, 41, 1 (in Chinese). (喻名强, 刘广义, 伊兴荣, 陈秦豫, 狄宁, 精细化工中间体, 2011, 41, 1.)
[13] (a) Liu, X. H.; Pan, L.; Tan, C. X.; Weng, J. Q.; Wang, B. L.; Li, Z. M. Pest. Biochem. Physiol. 2011, 101, 143. (b) Wu, R.; Zhu, C.; Du, X. J.; Xiong, L. X.; Yu, S. J.; Liu, X. H.; Li, Z. M.; Zhao, W. G. Chem. Cent. J. 2012, 6, 99.
[14] Liu, X. H.; Tan, C. X.; Weng, J. Q. Phosphorus, Sulfur, Silicon Relat. Elem. 2011, 186, 558.
/
| 〈 |
|
〉 |