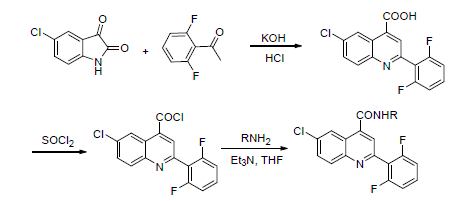
| [1] Liu, X. H.; Xu, X. Y.; Tan, C. X.; Weng, J. Q.; Xin, J. H.; Chen, J. Pest Manage Sci. 2015, 71, 292.
[2] Liu, X. H.; Sun, Z. H.; Yang, M. Y.; Tan, C. X.; Weng, J. Q.; Zhang, Y. G.; Ma, Y. Chem. Biol. Drug Des. 2014, 84, 342.
[3] Nicholson, A. N.; Stone, B. M.; Clarke C. H. J. Clin. Pharmacol. 1977, 4, 567.
[4] Zayed, M. E. M.; El-Shishtawy, R. M.; Elroby, S. A.; Obaid, A. Y.; Al-amshany, Z. M. Int. J. Mol. Sci. 2015, 16, 3804.
[5] Wang, L.; ?witalska, M.; Wang, N.; Du, Z.-J.; Fukumoto, Y.; Diep, N. K.; Kiguchi, R.; Nokami, J.; Wietrzyk, J.; Inokuchi, T. Molecules 2014, 19, 19021.
[6] Fragoulis, G.; Merli, A.; Reeves, G.; Meregalli, G.; Stenberg, K.; Tanaka, T.; Capri, E. Pest Manage. Sci. 2011, 67, 656.
[7] Liu, C. L. World Pesticide: Fungicide, Chemical Industry Press, Beijing, 2006, p. 251 (in Chinese). (刘长令, 世界农药大全杀菌剂卷, 化学工业出版社, 北京, 2006, p. 251.)
[8] Nakano, H.; Masumizu, T. JP 2009091320, 2009 [Chem. Abstr. 2010, 150, 472698].
[9] Hackler, R. E.; Jourdan G. P.; Johnson, P. L.; Thoreen, B. R.; Samaritoni, J. G. WO 9304580, 1993 [Chem. Abstr. 1993, 119, 139111].
[10] (a) Liu, X. H.; Weng, J. Q.; Wang, B. L.; Li, Y. H.; Tan, C. X.; Li, Z. M. Chem. Res. Intermed. 2014, 40, 2605. (b) Yang, M. Y.; Zhao, W.; Sun, Z. H.; Tan, C. X.; Weng, J. Q.; Liu, X. H. Lett. Drug Des. Discovery 2015, 12, 314. (c) Xie, F.; Liu, T. T.; Yang, G.; Yuan, J.; Kong, X. L.; Xu, T. M.; Tan, C. X. Chin. J. Org. Chem. 2013, 33, 2596 (in Chinese). (谢峰, 刘婷婷, 杨果, 袁静, 孔小林, 许天明, 谭成侠, 有机化学, 2013, 33, 2596.)(d) Yan, S. L.; Yang, M. Y.; Sun, Z. H.; Min, L. J.; Tan, C. X.; Weng, J. Q.; Wu, H. K.; Liu, X. H. Lett. Drug Des. Discov. 2014, 11, 940. (e) Sun, G.-X.; Sun, Z.-H.; Yang, M.-Y.; Liu, X.-H.; Ma, Y.; Wei, Y.-Y. Molecules 2013, 18, 14876.
[11] Karen, L.; Daniel, D. S. Synthesis 1993, 993.
[12] Yu, M. Q.; Liu, G. Y.; Y, X. R.; Chen, Q. Y.; Di, N. Fine Chem. Intermed. 2011, 41, 1 (in Chinese). (喻名强, 刘广义, 伊兴荣, 陈秦豫, 狄宁, 精细化工中间体, 2011, 41, 1.)
[13] (a) Liu, X. H.; Pan, L.; Tan, C. X.; Weng, J. Q.; Wang, B. L.; Li, Z. M. Pest. Biochem. Physiol. 2011, 101, 143. (b) Wu, R.; Zhu, C.; Du, X. J.; Xiong, L. X.; Yu, S. J.; Liu, X. H.; Li, Z. M.; Zhao, W. G. Chem. Cent. J. 2012, 6, 99.
[14] Liu, X. H.; Tan, C. X.; Weng, J. Q. Phosphorus, Sulfur, Silicon Relat. Elem. 2011, 186, 558. |