

由氨基酸衍生α-重氮膦酸酯不对称合成β-氨基膦酸酯衍生物反应研究
收稿日期: 2015-04-23
修回日期: 2015-06-02
网络出版日期: 2015-06-12
基金资助
天津市自然科学基金(No. 15JCYBJC20700)和新疆特色药食用植物资源化学实验室开放课题(No. 2015KL030)资助项目.
Asymmetric Synthesis of β-Amino Phosphonate Derivatives throughNatural Amino Acids Derived α-Diazophosphonates
Received date: 2015-04-23
Revised date: 2015-06-02
Online published: 2015-06-12
Supported by
Project supported by the Committee of Science and Technology of Tianjin City (No. 15JCYBJC20700) and the Xinjiang Laboratory of Native Medicinal and Edible Plant Resources Chemistry Open Subject (2015KL030).
胡辰飞 , 蔡岩 , 木尼热· , 阿布都克力木 , 苗志伟 . 由氨基酸衍生α-重氮膦酸酯不对称合成β-氨基膦酸酯衍生物反应研究[J]. 有机化学, 2015 , 35(10) : 2135 -2141 . DOI: 10.6023/cjoc201504035
An efficient procedure was developed for the asymmetric synthesis of (S)-diethyl (2-(1,3-dioxoisoindolin-2-yl)propyl)phosphonates employing (S)-diethyl (1-diazo-2-(1,3-dioxoisoindolin-2-yl)alkyl)phosphonates as starting materials. The reaction was catalyzed by 5% Pd/C and used glacial acetic acid as additive in i-PrOH as solvent. The obtained optical β-amino phosphonate derivatives were identified by 1H NMR, 13C NMR, 31P NMR and HRMS and the enantiomeric excess was 95%. This process provided straightforward access to optical β-aminophosphonate derivatives in good yields with high stereoselectivities.
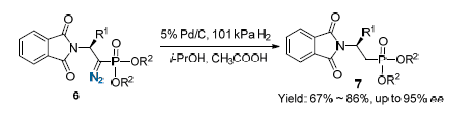
[1] (a) Ma, J. A. Chem. Soc. Rev. 2006, 35, 630.(b) Smith, W. W.; Barlett, P. A. J. Am. Chem. Soc. 1998, 120, 4622.(c) Hirschmann, R.; Smith, A. B., III; Taylor, C. M.; Benkovic, P. A.; Taylor, S. D.; Yager, K. M.; Sprengeler, P. A.; Bekovic, S. J. Science 1994, 265, 234.
[2] For recent review see the following: (a) Palacios, F.; Alonso, C.; de los Santos, J. M. Chem. Rev. 2005, 105, 899.(b) Palacios, F.; Alonso, C.; de los Santos, J. M. In Enantioselective Synthesis of β-Amino Acids, 2 ed., Eds.: Juaristi, E.; Soloshonok, V. A., Wiley, New York, 2005, p. 277.
[3] (a) Bartlett, P. A.; Hanson, J. E.; Morgan, B. P.; Ellsworth, B. A. In Synthesis of Peptides and Peptidomimetics, Houben-Weyl Methods in Organic Chemistry, 4th ed., Eds.: Goodman, M.; Toniolo, C.; Moroder, L.; Felix, A., Georg Thieme Verlag, 2004, Vol. E22c, p. 492.(b) Kukhar V. P.; Hudson, H. R. Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity, Wiley, New York, 2000.
[4] Takuya, H.; Keiji, M. J. Am. Chem. Soc. 2007, 129, 10054.
[5] Palaci, F.; Alonso, C.; Santos, J. M. Chem. Rev. 2005, 105, 899.
[6] Allen, J. G.; Arthenton, F. R.; Hall, M. J.; Hassall, C. H.; Holmes, S. W.; Lambert, R. W.; Nisbet, L. J.; Ringrose, P. S. Nature 1978, 272, 56.
[7] Smith, W. W.; Bartlett, P. A. J. Am. Chem. Soc. 1998, 120, 4622.
[8] Allen, M. C.; Fuhrer, W.; Tuck, B.; Wade, R.; Wood, J. M. J. Med. Chem. 1989, 32, 1652.
[9] Kong, S. S.; Fan, W. D.; Wu, G. P.; Miao, Z. W. Angew. Chem., Int. Ed. 2012, 51, 8864.
[10] Duggan, M. E.; Karanewsky, D. S. Tetrahedron Lett. 1983, 24, 2935.
[11] Smith, E. C. R.; Mcquaid, L. A.; Paschal, J. W.; DeHoniesto, J. J. Org. Chem. 1990, 55, 4472.
[12] Soloshonok, V. A.; Belokon, Y. N.; Kuzmina, N. A.; Maleev, V. I.; Svistunova, N. Y.; Solodenko, V. A.; Kukhar, V. P. J. Chem. Soc., Perkin Trans. 1 1992, 1525.
[13] Pachamuthu, K.; Schmidt, R. R. Chem. Commun. 2004, 1078.
[14] Wang, J.; Heikkinen, L. D.; Li, H., Zu, L. S.; Jiang, W.; Xie, H. X.; Wang, W. Adv. Synth. Catal. 2007, 349, 1052.
[15] Zhang, D.; Yuan, C. Chem.-Eur. J. 2009, 15, 4088.
[16] Cai, Y.; Lu, Y. C.; Yu, C. B.; Lyu, H. R.; Miao, Z. W. Org. Biomol. Chem. 2013, 11, 5491.
/
| 〈 |
|
〉 |